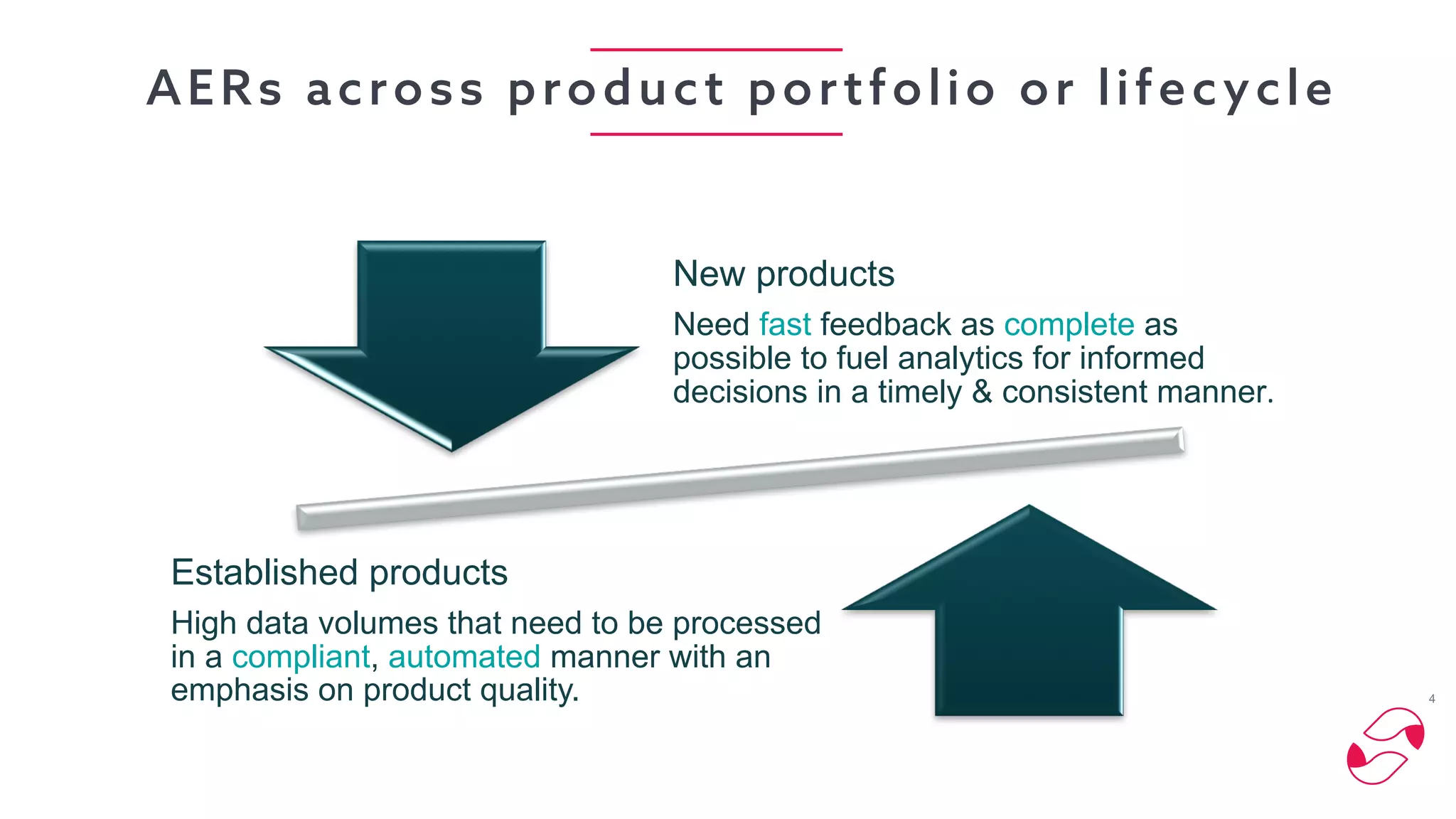
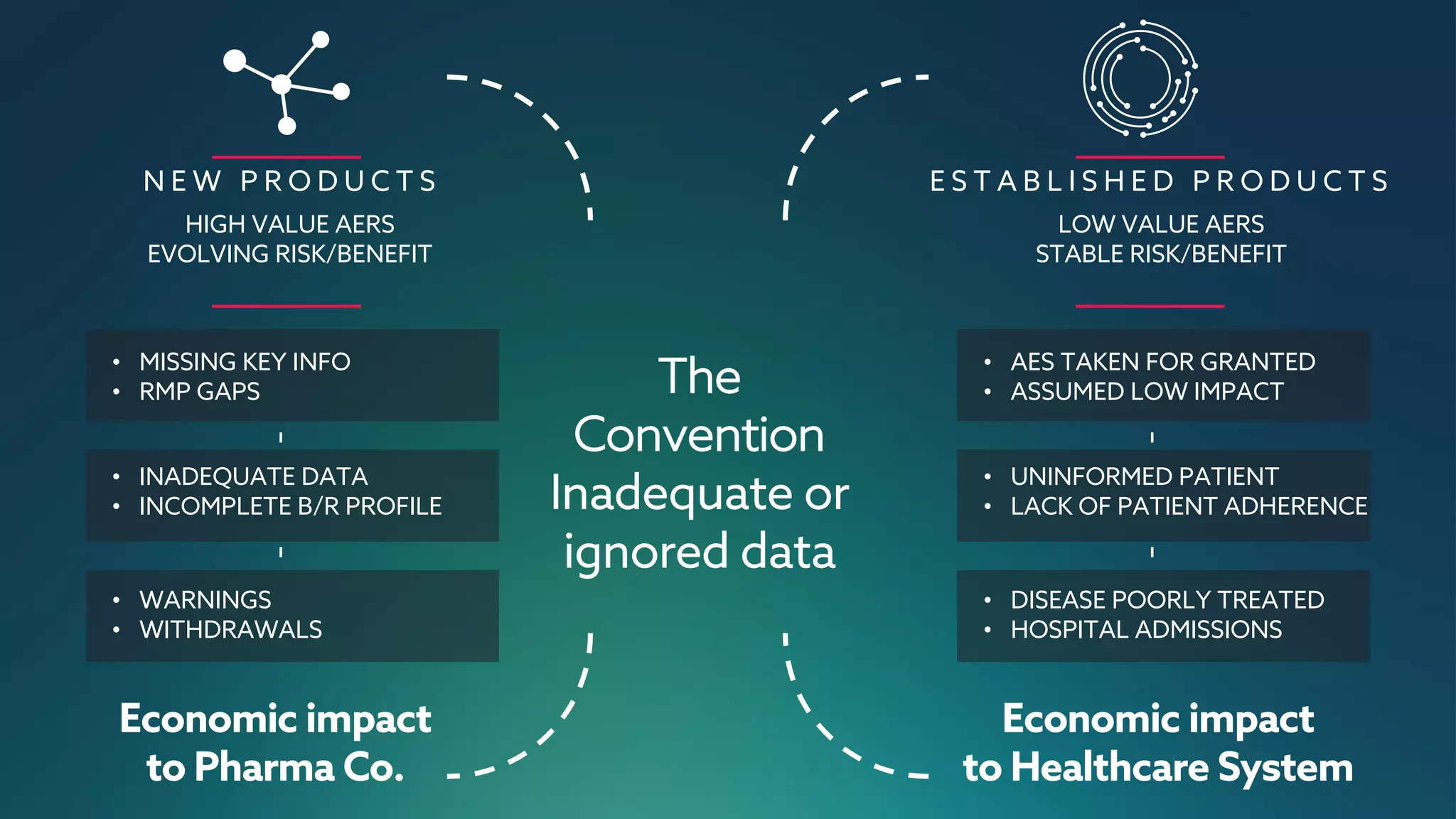
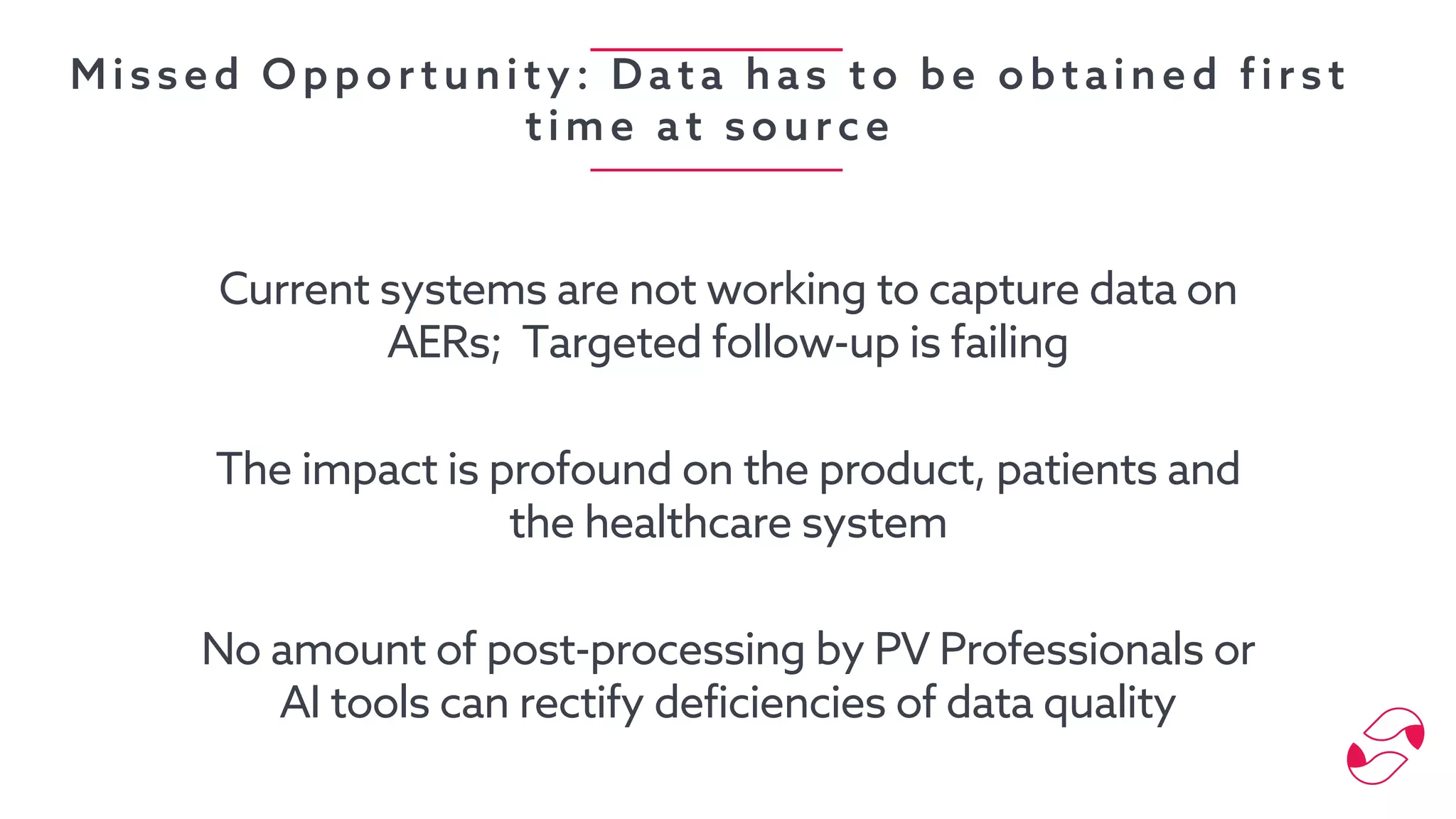
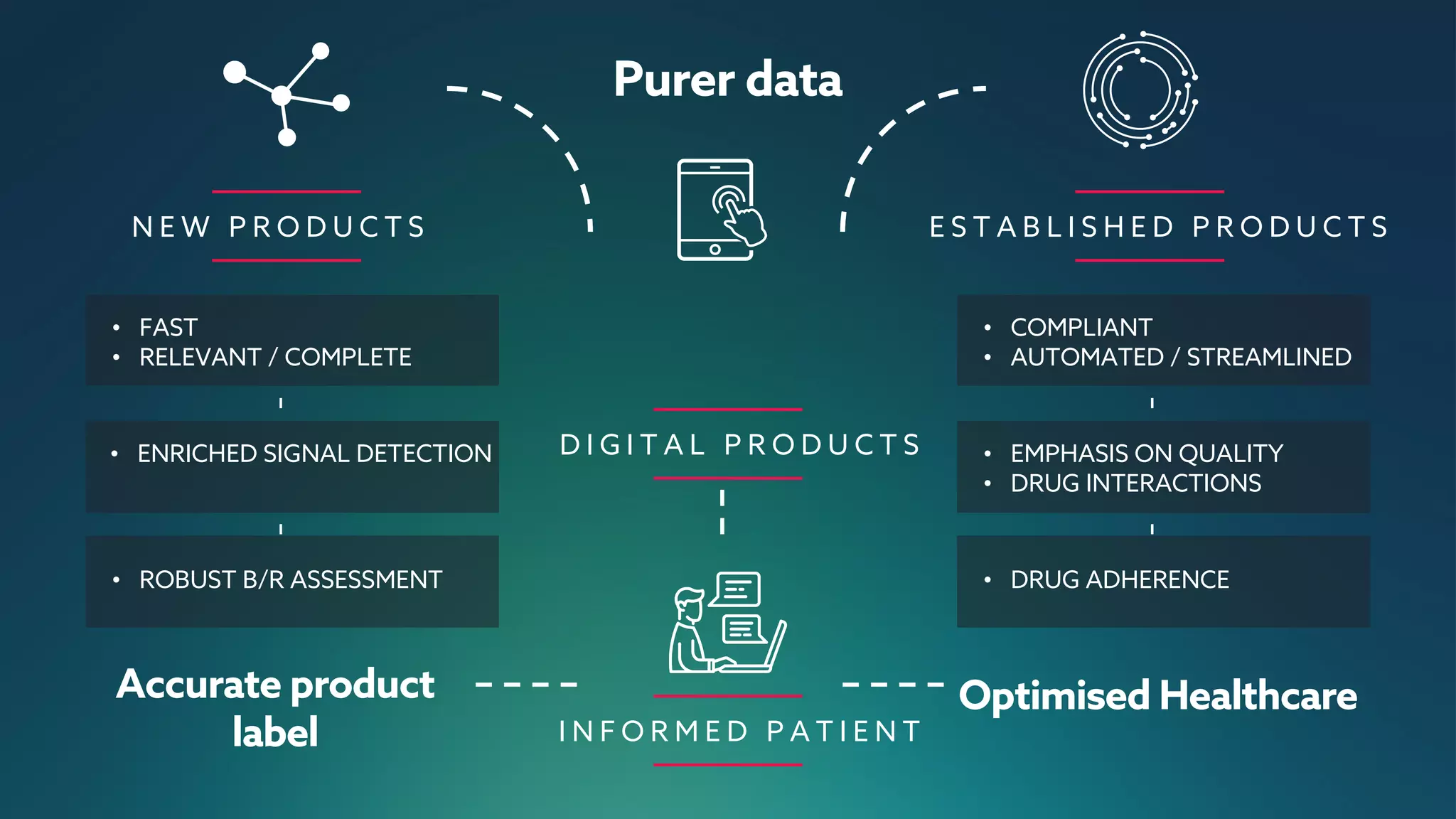
The document discusses the importance of digital innovation in healthcare, focusing on improving data quality and patient adherence through streamlined processes and targeted follow-up. It highlights the adverse drug events (ADEs) as a significant challenge, stressing that current systems fail to capture essential data during initial interactions. The use of digital tools like Reportum is emphasized to enhance data accuracy, improve regulatory compliance, and ultimately enhance patient safety and treatment efficiency.