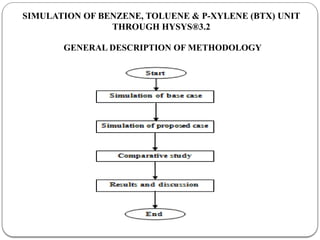
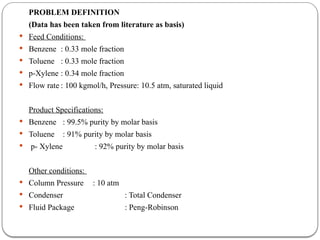
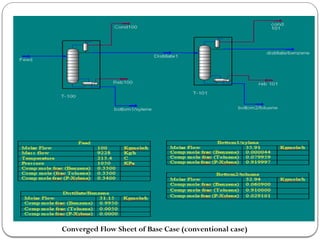
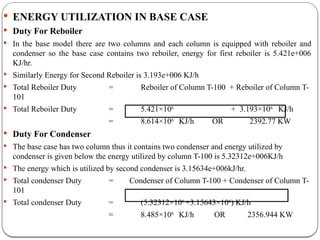
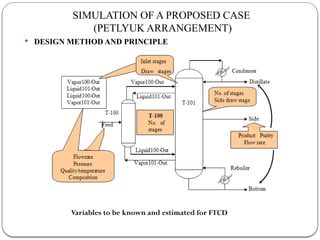
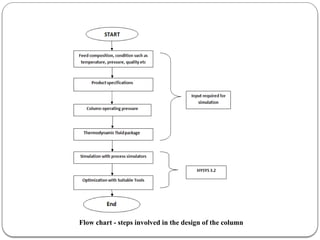
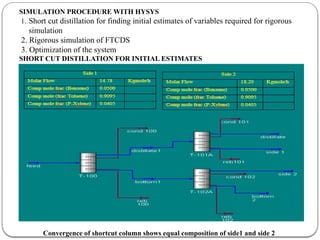
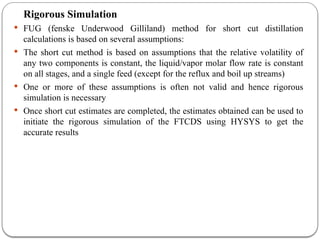
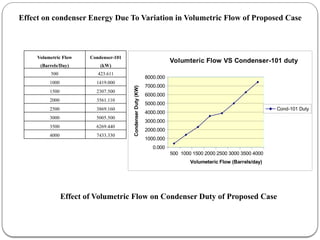
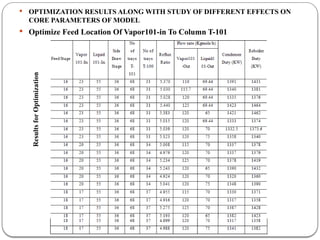
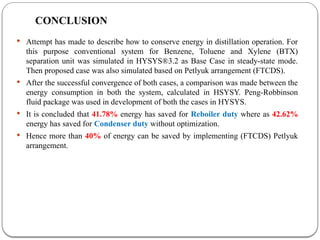
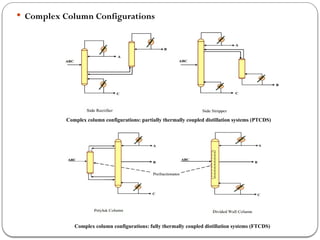
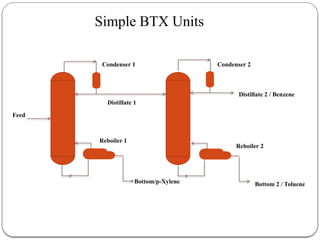
The document presents a design study on an energy-efficient distillation column using HYSYS® 3.2, focusing on the simulation of a base case vs. a proposed Petlyuk arrangement for BTX separation. It emphasizes the necessity for energy conservation in chemical industries due to rising energy costs and environmental issues, detailing various optimization techniques for distillation processes. The study includes literature reviews, methodology, simulation results, and discussions on energy utilization and material balances, showcasing significant differences in energy consumption between the conventional and proposed designs.



![LITERATURE REVIEW
Literature review forms an important part of research which helps to kick start the
research with valuable flow of information from the past research activities .
It is very much necessary to understand the background of Petlyuk arrangement for
this purpose literature has been reviewed.
The review reveals the existence of the technology, usage and acceptance of
Petlyuk arrangement (FTCDS) concept and recent developments in the technology.
Published Work On Petlyuk Arrangement
Hernandez et al (2003) gave the concept about Design and energy performance of
alternative schemes of Petlyuk distillation system He used three types of thermally
coupled distillation systems namely the sequence with side rectifier, the sequence
with side stripper and the Petlyuk column .
Ivor et al [2011] gave the concept of energy efficient distillation, according to them
distillation is responsible for a significant amount of energy consumption of the
world process industry. He said that The fully thermally coupled dividing wall
column has the attractive feature of both savings in energy consumption and
reduction of investment cost.](https://image.slidesharecdn.com/designofefficientdistillationcolumn-250113034618-e3074a39/85/Design-of-Efficient-Distillation-Column-pptx-11-320.jpg)
![ Salvador et al [1999] gave the concept about design of energy efficient Petlyuk system;
in their paper the energy efficient design of FTCDS (Petlyuk system) was presented.
Amminudin et al [2001] was done the work on design and optimization of Petlyuk
arrangement . He stated that the design of Petlyuk arrangement is more complex than
conventional arrangements because of the greater number of degrees of freedom.
Salvador et al [1999] worked on controllability and analysis of thermally coupled
distillation system. He did the comparison of the controllability properties of TCDCS
(Petlyuk, sequence with side rectifier, and sequence with side stripper) using singular
value decomposition was developed.
Amiya K.jana et al [2010] gave the concept about heat integrated distillation operation.
The heat integrated distillation has been researched for a number of decades.
Rong et al [2006] gave new heat integration configuration for Petlyuk arrangement i.e.
The Petlyuk arrangement had been proved to require the minimum energy consumption
for multicomponent distillation that is an advantage for saving both energy and capital
costs.](https://image.slidesharecdn.com/designofefficientdistillationcolumn-250113034618-e3074a39/85/Design-of-Efficient-Distillation-Column-pptx-12-320.jpg)
![ Michele et al [2007] worked on design of heat integrated distillation system for light
ends separation plant. The distillation systems employ the thermal coupling and the
heat integration principles to significantly reduce the heat requirements with respect
to the traditional simple column train.
Premkumar et al [2009] worked on (DWC), which works on the basis of (FTCDS),
was chosen for this study due to its lower energy consumption compared to the
conventional column system.
Malinen et al [2007] presented a method of rigorous minimum energy calculation
for non-ideal multicomponenet distillation. The method was based on column
simulation with a large number of equilibrium stages to mimic infinitely high
columns.](https://image.slidesharecdn.com/designofefficientdistillationcolumn-250113034618-e3074a39/85/Design-of-Efficient-Distillation-Column-pptx-13-320.jpg)