DermatologyTimesJune2015
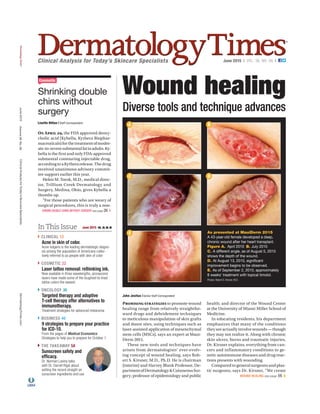
- 1. CLINICAL 13 Acne in skin of color. Acne vulgaris is the leading dermatologic diagno- sis among the population of Americans collec- tively referred to as people with skin of color COSMETIC 22 Laser tattoo removal: rethinking ink. Now available in three wavelengths, picosecond lasers have made some of the toughest-to-treat tattoo colors the easiest ONCOLOGY 36 Targeted therapy and adoptive T-cell therapy offer alternatives to immunotherapy. Treatment strategies for advanced melanoma BUSINESS 46 9 strategies to prepare your practice for ICD-10. From the pages of Medical Economics: Strategies to help you to prepare for October 1 THE TAKEAWAY 58 Sunscreen safety and efficacy. Dr. Norman Levine talks with Dr. Darrell Rigel about setting the record straight on sunscreen ingredients and use Clinical Analysis for Today’s Skincare Specialists June 2015 | VOL. 36, NO. 06 | Shrinking double chins without surgery Lisette Hilton | Staff Correspondent In This Issue June 2015 VOL. 36, NO. 06 John Jesitus | Senior Staff Correspondent Wound healing P to promote wound healing range from relatively straightfor- ward drugs and debridement techniques to meticulous manipulation of skin grafts and donor sites, using techniques such as laser-assisted application of mesenchymal stem cells (MSCs), says an expert at Maui- Derm 2015. These new tools and techniques have arisen from dermatologists’ ever-evolv- ing concept of wound healing, says Rob- ert S. Kirsner, M.D., Ph.D. He is chairman (interim) and Harvey Blank Professor, De- partmentofDermatology&CutaneousSur- gery; professor of epidemiology and public health; and director of the Wound Center at the University of Miami Miller School of Medicine. In educating residents, his department emphasizes that many of the conditions theyseeactuallyinvolvewounds—though they may not realize it. Along with chronic skin ulcers, burns and traumatic injuries, Dr. Kirsner explains, everything from can- cers and inflammatory conditions to ge- netic autoimmune diseases and drug reac- tions presents with wounding. Compared to general surgeons and plas- tic surgeons, says Dr. Kirsner, “We create WOUND HEALING see page 16 O A , the FDA approved deoxy- cholic acid (Kybella, Kythera Biophar- maceuticals)forthetreatmentofmoder- ate-to-severesubmentalfatinadults.Ky- bella is the first and only FDA-approved submental contouring injectable drug, accordingtoaKytherarelease.Thedrug received unanimous advisory commit- tee support earlier this year. Helen M. Torok, M.D., medical direc- tor, Trillium Creek Dermatology and Surgery, Medina, Ohio, gives Kybella a thumbs up. “For those patients who are weary of surgical procedures, this is truly a non- SHRINK DOUBLE CHINS WITHOUT SURGERY see page 26 Cosmetic Diverse tools and technique advances A C E B D As presented at MauiDerm 2015 A 43-year-old female developed a deep, chronic wound after her heart transplant. Figure A. April 2010 B. July 2010 C. A different angle, as of August 5, 2010 shows the depth of the wound. D. At August 13, 2010, significant improvement begins to be observed. E. As of September 2, 2010, approximately 8 weeks’ treatment with topical timolol. Photos: Robert S. Kirsner, M.D. DermatologyTimes® June2015Volume36No.06ClinicalAnalysisforToday’sSkincareSpecialistsDermatologyTimes.com ES618881_DT0615_CV1.pgs 05.22.2015 23:11 ADVblackyellowmagentacyan
- 2. Harness the power ®/TMs are trademarks of Valeant Pharmaceuticals International, Inc., or its affiliates. Any other product or brand names and logos are the property of their respective owners. Distributed by OMP, Inc. ©2015 Obagi Medical, a division of Valeant Pharmaceuticals North America LLC. DM/OBG/14/0096(1)c(2) 04/15 www.obagi.com The power of Obagi is backed by science, resulting in clinically proven products that can transform the look of your patients’ skin. For more information, call 1.800.636.7546 today. ES615052_DT0615_CV2_FP.pgs 05.16.2015 02:44 ADVblackyellowmagentacyan
- 3. 3JUNE 2015 ⁄ DERMATOLOGYTIMES.COM EDITORIAL ADVISORY BOARD Insight & Opinion From Our Advisory Board Leaders W e are entering a new era in dermatology where numer- ous drugs are available with the sole purpose of improving appear- ance and not treating disease. Certainly, aging could be considered a disease that affects all humans, but it is possible to argue that since all humans age, it is normal and not a disease. Whether the appearance degrada- tion of aging is normal or a disease, the neurotoxins that are being used to treat the condition are considered drugs and are studied and examined for safety in the same manner as all drugs designed to treat disease. This is exemplified by most recent draft guidance issued in August 2014 by the FDA on Upper Facial Lines: Developing Botulinum Toxin Drug Products. This guidance states that for a neurotoxin to be approved as effica- cious, it must remove all motion in the injection area receiving a rating in the category of “none” or zero. While this is quite desirable in the glabellar area, where frowning is almost always con- sidered an unattractive facial expres- sion, it might not be desirable to elimi- nate all movement around the eyes, a common area of neurotoxin injection. Many have observed that failure of skin movement around the eyes when smil- ing leads to the perception by others of insincerity. Indeed, some contraction of the skin around the eyes when smil- ing is normal since the lower eye con- tracts simultaneously with the corner of the mouth, which produces lifting of the mouth corner and smiling. NEED FOR NEW CRITERIA Dermatologic drugs are studied on an ordinal scale of 0 to 4 where 0 = clear, 1 = almost clear, 2 = mild, 3 = moderate, and 4 = severe. This scale works quite well for the evaluation of acne or psoria- sis medications where clear or almost clear is the standard by which excellent drugs should be judged. I am not sure this same scale works for the evalua- tion of new neurotoxins hoping for ap- proval. It might be that the reduction of periorbital movement and wrinkling from moderate to mild would be superior to the reduction of periorbital wrinkling from moderate to none. No movement is not aesthetically pleasing and aesthetic assessment is a much different realm than drug treatment success. There is a need for new evaluation criteria for drugs that function in the aesthetic realm. The same ordinal scale can be used, but the ordinal rating required for success and subsequent drug approval must be reconsidered. With neurotoxins, wrinkles in some loca- tions are good while wrinkles in other locations are bad. Foreheads that are shiny and eyebrows that cannot elevate are dead giveaways that chemodener- vation is at work. As a matter of fact, the difference in appearance from the totally paralyzed forehead 1 week after injection to the partially moving fore- head 2 months after injection to the total moving forehead 3 months after injec- tion eliminates any doubts that the ap- pearance change is natural. It is obvious that neurotoxins are at work! There is certainly something to be said for projecting natural good looks as opposed to the sometimes contorted appearance created by neurotoxins. Using some neurotoxins that eliminate movement and others that simply de- crease movement could sculpt a more natural appearance. For example, it would be beneficial to paralyze the frown with an aggressive toxin appli- cation, but the fine lines on the lateral cheeks that appear with smiling could be softened with a milder toxin effect. I liken the facial use of neurotoxins to painting a portrait. Using oil pigments on canvas to capture the essence of a beautiful face takes more than one brush. Imagine if Leonardo da Vinci used one large brush to paint the Mona Lisa! The outcome would have been much different. Similarly, the dermatolo- gist artist crafting a beautiful face also needs more than one toxin with different degrees of denervation to achieve suc- cess. I think cosmetic dermatology must revisit the “none” or “almost none” phenomenon for treatment success. None or almost none has tremendous importance when addressing drugs where success is defined as cure. It is not really possible to cure wrinkles other than by early death, which clearly would not defined as treatment suc- cess. Perhaps for drugs that address appearance issues, a new ordinal static scale should be developed that is clinically meaningful from an aesthetic standpoint. DT Cosmetic dermatology and the ‘none’ or ‘almost none’ phenomenon It is not really possible to cure wrinkles other than by early death, which clearly would not be defined as treatment success... a new ordinal static scale should be developed. Zoe Diana Draelos, M.D., is a clinical and research dermatologist with Dermatology Consulting Services, High Point, N.C. and a consulting professor of dermatology, Duke University School of Medicine, Durham, N.C. ES615717_DT0615_003.pgs 05.19.2015 21:25 ADVblackyellowmagentacyan
- 4. ® JUNE 2015 ⁄ DERMATOLOGYTIMES.COM 4 EDITORIAL ADVISORY BOARD Let your voice be heard, contact us: editor@dermatologytimes.com DermatologyTimesistheonlyclinicalnewsresourceserving areadershipofmorethan14,000dermatologistsandother professionalsfocusedonskincare.Throughunbiased reporting,westrivetohelppractitionersputintoperspective developmentsthataffecttheirbusiness.Ourgoalistoprovide practicalinformationthatwillhelpthemtobetterunderstand clinical,regulatoryandfinancialissues,aswell aschartbusinessgrowth. Our Mission The Dermatology Times Editorial Advisory Board qualifies the editorial content of the magazine. Members review meeting programs; suggest story topics, special reports and sources; evaluate manuscripts; conduct interviews and roundtables; and counsel editors as questions arise. Dr. Joel Schlessinger Omaha, Neb. Dr. James Spencer St. Petersburg, Fla. Dr. Helen Torok Medina, Ohio Dr. Philip Werschler Spokane, Wash. Dr. Albert Yan Philadelphia, Pa. Dr. Tina Alster WashingtonD.C. Dr. Seth Matarasso San Francisco, Calif. Dr. Patti Farris New Orleans, La. Dr. Roy Geronemus New York, N.Y. Dr. David Goldberg New York, N.Y. Dr. Ranella Hirsch Boston, Mass. Zoe Diana Draelos, M.D., is consulting professor of dermatology, Duke University School of Medicine, Durham, N.C. Norman Levine, M.D., is a private practitioner in Tucson, Ariz. Ronald G. Wheeland, M.D., is a private practitioner in Tucson, Ariz. Elaine Siegfried, M.D., is professor of pediatrics & dermatology, Saint Louis University Health Sciences Center, St. Louis, Mo. PRINTED IN U.S.A. content CONTENT CHANNEL DIRECTOR Heather Onorati } (440)826-2868|honorati@advanstar.com CONTENT MANAGING EDITOR Pamela Kreigh }(440)891-2610|pkreigh@advanstar.com AESTHETIC CONTENT EDITOR Eliza Cabana }(440)891-2671|eliza.cabana@advanstar.com CONTENT SPECIALIST Annamarie Iannetta }(440)891-2606|aIannetta@advanstar.com COSMETIC COLUMNIST Zoe Diana Draelos, M.D. LASER & LIGHT DEVICES COLUMNIST Joely Kaufman, M.D. LEGAL AFFAIRS COLUMNIST David J. Goldberg, M.D., J.D. GROUP ART DIRECTOR Robert McGarr } rmcgarr@advanstar.com ART DIRECTOR Lecia Landis } llandis@advanstar.com SENIOR PRODUCTION MANAGER Karen Lenzen } (218)740-6371|klenzen@media.advanstar.com publishing & sales EVP Georgiann DeCenzo } gdecenzo@advanstar.com VP, GROUP PUBLISHER Ken Sylvia } (732)346-3017 ksylvia@advanstar.com PUBLISHER Amy Ammon } (732)346-3089|cell:(845)521-6950 aammon@advanstar.com NATIONAL ACCOUNT MANAGER Diane Kebabjian } (732)346-3034|cell:(201)484-9754 dkebabjian@advanstar.com DIR. OF BUSINESS DEVELPMENT, Margie Jaxel } (732)-346-3003 HEALTHCARE TECHNOLOGY SALES mjaxel@advanstar.com ACCOUNT MANAGER, Karen Gerome }(440)891-2670 CLASSIFIED/ DISPLAY ADVERTISING kgerome@advanstar.com ACCOUNT MANAGER, Joanna Shippoli } 440-891-2615 RECRUITMENT ADVERTISING jshippoli@advanstar.com BUSINESS DIRECTOR, EMEDIA Don Berman } (212) 951-6745 dberman@advanstar.com DIRECTOR OF MARKETING & Gail Kaye } (732)346-3042 RESEARCH SERVICES gkaye@advanstar.com SALES SUPPORT Hannah Curis } (732)346-3055 hcuris@advanstar.com LIST ACCOUNT EXECUTIVE Renee Schuster } (440)891-2613 rschuster@advanstar.com PERMISSIONS Maureen Cannon } (440)891-2742 mcannon@advanstar.com REPRINTS Inquiries involving reprints should be directed to 877-652-5295 ext. 121 bkolb@wrightsmedia.com Outside US, UK, direct dial: 281-419-5725. ext. 121 audience development CORPORATE DIRECTOR Joy Puzzo } jpuzzo@advanstar.com DIRECTOR Christine Shappell } cshappell@advanstar.com SUBSCRIPTIONS INQUIRIES, including changes of address, should be directed to (877) 922-2022 or (218) 740-6477. UBM Advanstar CHIEF EXECUTIVE OFFICER Joe Loggia EVP, LIFE SCIENCES Tom Ehardt EVP Georgiann DeCenzo EVP Chris DeMoulin EVP, BUSINESS SYSTEMS Rebecca Evangelou EVP, HUMAN RESOURCES Julie Molleston EVP, STRATEGY & BUSINESS DEVELOPMENT Mike Alic SR VICE-PRESIDENT Tracy Harris VP, GM PHARM/SCIENCE GROUP Dave Esola VP, LEGAL Michael Bernstein VP, MEDIA OPERATIONS Francis Heid VP, TREASURER & CONTROLLER Adele Hartwick UBM Americas CHIEF EXECUTIVE OFFICER Sally Shankland CHIEF OPERATING OFFICER Brian Field CHIEF FINANCIAL OFFICER Margaret Kohler UBM plc CHIEF EXECUTIVE OFFICER Tim Cobbold GROUP OPERATIONS DIRECTOR Andrew Crow CHIEF FINANCIAL OFFICER Robert Gray CHAIRMAN Dame Helen Alexander ES616316_DT0615_004.pgs 05.20.2015 04:34 ADVblackyellowmagentacyan
- 5. Finacea® Foam will be in the picture (azelaic acid) Foam,15% © 2015 Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ 07981. Bayer, the Bayer Cross, and Finacea are registered trademarks of Bayer. All rights reserved. PP-825-US-0318 June 2015 www.finaceafoam.com ES621799_DT0615_005_FP.pgs 05.27.2015 02:38 ADVblackyellowmagentacyan
- 6. Dermatology Times App Get access to all the benefits Dermatology Times offers at your fingertips. The Dermatology Times app for iPad & iPhone is now free in the iTunes store.Dermatology Times is part of the ModernMedicine Network, a Web-based portal for health professionals offering best-in-class content and tools in a rewarding and easy-to-use environment for knowledge-sharing among members of our community. facebook.com/DermatologyTimes Like us on Facebook and participate in the discussion @doctorclaudia Hi-res images of #acne lesions http:// buff.ly/1R9tuWB #Dermatology via @ DermTimesNow @MDAMelanomaOnc MD Anderson #Melanoma Moon Shot cited: Innovative programs for #skinc @ducrest Here is a need for new evaluation criteria for drugs that function in the aesthetic realm via @DermTimesNow practice exit strategy: spr.ly/60190V2V @DrRothaus #Psoriasis impact goes more than skin deep: managing psychosocial burden is just as important @DermTimesNow bit.ly/1IbXPBe Follow us on Twitter to receive the latest news and participate in the discussion twitter.com/DermTimesNow How to improve appearance of cracked heels What’s your diagnosis? Blog A 48-year-old man with a 2-year history of painful, disseminated, tender nodules covering his scalp. CHOOSE ONE: HIDRADENITIS SUPPURATIVA FURUNCULOSIS FOLLICULITIS bit.ly/junediagnosis Photos:ImageappearswithpermissionfromVisualDx.LogicalImagesInc. Zoe Diana Draelos, MD bit.ly/improvecrackedheels LAST MONTH’S DIAGNOSIS: Actinic cheilitis bit.ly/maydiagnosisLearn more at: VIDEOS E-mazing medical marketing minute AdamDeGraide CEOatCrystalClear DigitalMarketing, LLC,sitsdownwith Dr.RobertMarshall, medicaldirector atRejuvenateMedSpa.Theydiscussthevalue ofproperlybrandingamedicalpracticeonline, includingdesigningawebsite,logosandmuch more. bit.ly/E-mazing-June-Interview Realistic patient expectations? Can you set realistic expectations for patients undergoing laser skin tightening? Laser Roundtable panelists share their answers at Vegas Cosmetic Surgery 2014. bit.ly/realisticexpectations JUNE 2015 ⁄ DERMATOLOGYTIMES.COM 6 INTER CTIVE Resource Centers ® For more information on specialized areas of dermatology, related articles and business resources, go to: modernmedicine.com/ResourceCenters Best practices in the evaluation and management of actinic keratoses DermatologyTimes.com/actinickeratoses Current and emerging therapies for psoriatic arthritis DermatologyTimes.com/psoriatic-arthritis Fillers and toxins: Cosmetic and therapeutic options DermatologyTimes.com/injectables Insights into managing atopic dermatitis and acne DermatologyTimes.com/atopicdermatitis ES615883_DT0615_006.pgs 05.20.2015 00:03 ADVblackyellowmagentacyan
- 8. References: 1. Stein Gold L, Kircik L, Fowler J, et al; Ivermectin Phase III Study Group. Efficacy and safety of ivermectin 1% cream in treatment of papulopustular rosacea: results of two randomized, double-blind, vehicle-controlled pivotal studies. J Drugs Dermatol. 2014;13(3):316-323. 2. Data on file. Galderma Laboratories, L.P. 3. Taieb A, Ortonne JP, Ruzicka T, et al; Ivermectin Phase III Study Group. Superiority of ivermectin 1% cream over metronidazole 0.75% cream in treating inflammatory lesions of rosacea: a randomized, investigator-blinded trial. Br J Dermatol. In press. All trademarks are the property of their respective owners. ©2015 Galderma Laboratories, L.P. Galderma Laboratories, L.P. 14501 N. Freeway, Fort Worth, TX 76177 IVM-143B Printed in USA 02/15 BRIEF SUMMARY This summary contains important information about SOOLANTRA (soo lan’ trah) Cream. Read this information carefully before you prescribe SOOLANTRA Cream. For full Prescribing Information and Patient Information please see the package insert. WHAT IS SOOLANTRA CREAM? SOOLANTRA Cream is a topical prescription medicine indicated for the treatment of the inflammatory lesions of rosacea. WHO IS SOOLANTRA CREAM FOR? SOOLANTRA Cream is indicated for people with inflammatory lesions of rosacea. It is not known if SOOLANTRA Cream is safe and effective for children. Advise your patients to not use SOOLANTRA Cream for a condition for which it was not prescribed and remind them to not give SOOLANTRA Cream to other people, even if they have the same symptoms as it may harm them. WHAT SHOULD I ASK MY PATIENTS BEFORE PRESCRIBING SOOLANTRA CREAM? Before you prescribe SOOLANTRA Cream, ask your patients if they: A have any other medical conditions. A &6* 46*,2&28 36 40&22.2, 83 '*(31* 46*,2&28 8 .7 238 /23;2 .+ SOOLANTRA Cream can harm an unborn baby. A are breastfeeding or plan to breastfeed. It is not known if SOOLANTRA Cream passes into breast milk and if it can harm a baby. WHAT ARE THE MOST COMMON SIDE EFFECTS OF SOOLANTRA CREAM? The most commonly reported side effects when using SOOLANTRA Cream include skin burning sensation and skin irritation. Remind your patients to tell you if they have any side effect that bothers them or that does not go away. These are not all of the possible side effects of SOOLANTRA Cream. For more information, see the full Prescribing Information. You are encouraged to report negative side effects of prescription drugs to the FDA at www.fda.gov/medwatch or call 1-800-FDA-1088. You may also contact GALDERMA LABORATORIES, L.P. AT 1-866-735-4137. HOW SHOULD PATIENTS USE SOOLANTRA CREAM? A SOOLANTRA Cream is for use on the face only and should not be used in the eyes, mouth, or vagina. A SOOLANTRA Cream should be applied to the affected areas of the face once a day. APPLYING SOOLANTRA CREAM: A A pea-sized amount of SOOLANTRA Cream should be applied to each area of the face (forehead, chin, nose, each cheek) that is affected. Avoid contact with the lips and eyes. SOOLANTRA Cream is supplied in a child-resistant capped tube. A To open, gently press down on the child resistant cap and twist counterclockwise. To avoid spilling, do not squeeze the tube while opening or closing. A To close, gently press down on the child resistant cap and twist clockwise. WHAT ARE THE INGREDIENTS IN SOOLANTRA CREAM? Active ingredient: ivermectin. Inactive ingredients: carbomer copolymer type B, cetyl alcohol, citric acid monohydrate, dimethicone, edetate disodium, glycerin, isopropyl palmitate, methylparaben, oleyl alcohol, phenoxyethanol, polyoxyl 20 cetostearyl ether, propylene glycol, propylparaben, purified water, sodium hydroxide, sorbitan monostearate, and stearyl alcohol. WHERE SHOULD I GO FOR MORE INFORMATION ABOUT SOOLANTRA CREAM? A This Brief Summary summarizes the most important information about SOOLANTRA Cream. For full Prescribing Information and Patient Information please see the package insert. A 3 83 www.soolantra.com or call 1-866-735-4137 Trademarks are the property of their respective owners. GALDERMA LABORATORIES, L.P., Fort Worth, Texas 76177 USA Revised: December 2014 IMPORTANT INFORMATION ABOUT SOOLANTRA® (ivermectin) Cream, 1% ES615051_DT0615_008_FP.pgs 05.16.2015 02:44 ADVblackyellowmagentacyan
- 9. 9JUNE 2015 ⁄ DERMATOLOGYTIMES.COM LEGALEAGLE D r. Derm is a hard working der- matologist practicing in a city that has yet to fully recover from the recession. Overhead is up and reve- nues are down. He is left with no choice but to start terminating some employ- ees. He has 22 employees. He evaluates his employees and finds that they all per- form well. However, he is aware that a particular 27-year-old bulimic employee takes continuous breaks to eat small por- tions of food followed by prolonged bath- room breaks. Dr. Derm is annoyed with this behavior, feels it is disruptive to the work environment, and has decided it interferes with patient-staff flow. He termi- nates this employee. Before long he is served with a law suit. His former employee alleges that she was terminated for behaviors related to her bulimia. In the lawsuit, she contends that her eating behavior was protected under the Americans with Disabilities Act. Dr. Derm is now devastated. In his at- tempt to lessen the financial burdens of his practice, he ends up with a lawsuit. He seeks legal help. What is the Americans with Disabilities Act? Will Dr. Derm lose this lawsuit? ADA GENERAL LEGAL FRAMEWORK The Americans with Disabilities Act of 1990 (ADA) provides a general legal frame- work for access of individuals with disabili- ties to public places and also for accom- modating employees with disabilities in the workplace. Although the ADA contains very specific guidance for physical accom- modations, such as wheelchair access, it provides little guidance relevant to workers with conditions such as diabetes. The employment provision of the ADA applies to employers who have 15 or more employees. The gist of the ADA is that it bans discrimination against a “qual- ified individual with a disability.” This is defined as “an individual with a disabil- ity who, with or without reasonable ac- commodation, can perform the essential functions of the employment position that such an individual holds or hires.” The ADA also prohibits discrimination based on a perceived disability; that is, when the employer wrongly assumes an employee cannot do a job because of a disability. If employees are fired for legitimate work- related reasons, there cannot be an ADA claim, even if the employee happens to also have disability. The ADA defines disability as: “(A) a physical or mental impairment that sub- stantially limits one or more of the major life activities of such an individual; (B) a record of such impairment; (C) being re- garded as having such impairment.” The disability must also last at least 6 months, and not be a natural, self-limited condition such as pregnancy. The ADA does not protect those who engage in illegal use of drugs or the use of alcohol in the workplace. Employees with substance abuse problems may be held to the same standard of behavior as other employees. However, those who have successfully completed a rehabili- tation program for drugs, or who are cur- rently enrolled in a program and not cur- rently using drugs, are protected. Of note, courts have differed on whether alcohol- ism itself is a disability. Some federal cir- cuit courts say alcoholism is a disability, but others have ruled that it is not. IS BULIMIA A DISABILITY UNDER THE ADA? Better asked, does bulimia fit under the ADA requirement as an impairment that substantially limits a major life activity? In general, courts look at this definition quite closely. In Bragdon v. Abbott, the United States Supreme Court considered whether an asymptomatic disease — HIV — could be a disability under the ADA. The plaintiff’s dentist refused to fill a cav- ity in the plaintiff’s tooth in his office, as- serting that he did not have sufficient in- fection control equipment. The lawsuit was brought under the public facilities section of the ADA, but the test for dis- ability is the same as in the employment section. The Supreme Court first inquired into whether asymptomatic HIV infection was an impairment. The court held that the pro- found effects of HIV on the immune sys- tem qualify as an impairment, without re- quiring that the impairment produce symp- tomatic disease. The same ruling might apply to an asymptomatic bulimic — buli- mia being a complex disease that might ul- timately impact many organ systems. Having found that HIV is an impair- ment, the Court then asked whether the impairment could substantially limit a major life activity though not yet causing symptomatic illness. The court found that being infected with HIV would affect deci- sions about one’s major life activities and, in this plaintiff’s case, would affect the de- cision to have children. The same analy- sis might apply to a 27-year-old bulimic’s concern of the impact of this disease on her having and raising children. Nevertheless, in Salim v. MGM Grand Detroit, L.L.C., the court rejected the claim of a person with diabetes as being substantially limited in working, thinking and taking care of herself, noting that not being able to perform a job during a par- ticular time (i.e., time needed to eat) does not rise to the level of being substantially limited in the major life activity of working. Conversely, in Lawson v. CSX Transporta- tion, Inc., the court determined that a jury could reasonably find the plaintiff sub- stantially limited in the major life activity of eating as a result of his diabetes severely restricting what and when he could eat. It is totally understandable that in dif- ficult economic times, Dr. Derm wants to cut back on his staff. If his employees do not have a contract of employment, he can terminate an employee at will. How- ever, when he terminated his employee for her bulimia, he allowed himself the po- tential to be sued in violation of the ADA. A long, expensive trial with an ultimate jury decision may ensue. DT David Goldberg, M.D., J.D., is director of Skin Laser and Surgery Specialists of New York and New Jersey; director of laser research, Mount Sinai School of Medicine; and adjunct professor of law, Fordham Law School. I have been sued under the ADA ES615718_DT0615_009.pgs 05.19.2015 21:25 ADVblackyellowmagentacyan
- 10. ® JUNE 2015 ⁄ DERMATOLOGYTIMES.COM 10 IRREGULARBORDER R esveratrol is a naturally occur- ring polyphenolic antioxidant found in over 70 plant species. Common sources include grapes, pea- nuts and ripe berries. In nature, res- veratrol serves to protect plants from ultraviolet light, infections and other environmental stressors. Resveratrol is best known as the longevity molecule since scientific studies have demon- strated that resveratrol extends lifespan in a variety of animal species.1 The life-extending benefits of resver- atrol are attributed to the fact that, like caloric restriction, resveratrol modulates sirtuin gene expression.2 Sirtuins are a group of enzymes that influence impor- tant adaptive functions such as cell survival, DNA repair, gluconeogenesis, cell-cycle regulation, insulin sensitivity, lipid metabolism and fat mobilization.3 In addition to extending lifespan, res- veratrol has been touted to improve healthspan. Accordingly, resveratrol has been studied in almost all fields of med- icine and has significant potential value for treating a variety of disease states. Beneficial effects include neuroprotec- tion, cardioprotection, anti-diabetic and anti-tumor activity.4 Resveratrol also has broad anti- inflammatory effects and inhibits a variety of inflammatory markers includ- ing cox-2, IL-2, IL-6, IL-8 and vascu- lar endothelial growth factor.5 Ongoing studies are being conducted to confirm the health benefits of resveratrol. PHARMACOKINETICS OF RESVERATROL The clinical use of resveratrol has been limited by the challenges of oral admin- istration.6 Although 70% of orally admin- istered resveratrol is absorbed, it is rap- idly metabolized to glucuronide and sul- fated forms. Even after large oral doses, only trace amounts of resveratrol can be detected in the bloodstream. The health effects of the glucuronide and sulfated derivatives of resveratrol are yet to be determined. Topical formulation with resveratrol is equally challenging. Resveratrol readily isomerizes to the less desirable “cis” form when exposed to ultraviolet light.7 Another challenge is that resveratrol is a hydrophobic molecule, with a water sol- ubility estimated at 0.05 mg/mL, making it difficult to stabilize in meaningful con- centrations in formulation.8 In view of these challenges, it is not surprising that few high concentration formulations of resveratrol have been commercialized. SKIN AGING AND RESVERATROL Oxidative stress is known to contrib- ute to skin aging. Free radicals are produced as we age naturally and by extrinsic factors such as ultraviolet light, pollution and cigarette smoking.9 Reactive oxygen species (ROS) ac- cumulate in cells where they damage lipid membranes, proteins and DNA. Free radicals upregulate transcrip- tion factor activator protein 1 (AP-1) that turns on the synthesis of colla- gen digesting matrix metalloprotein- ases, reduces collagen content in skin and contributes to skin wrinkling. Oxi- dative stress also upregulates nuclear factor kappa beta (NF-kB), increasing the synthesis of a variety of inflamma- tory mediators that contribute to skin aging. For these reasons, the use of topical and systemic antioxidants are of value for prevention of skin aging. Resveratrol is unique among anti- oxidants in that it functions as an anti- oxidant in several ways.10 It serves as a free radical scavenger that effectively quenches reactive oxygen species such as hydroxyl, superoxide and metal- induced radicals. Patricia K. Farris, M.D. is owner of Old Metairie Dermatology, Metairie, La., Clinical Associate Professor at Tulane University, and editor of the textbook Cosmeceuticals and Cosmetic Practice. Resveratrol, the longevity molecule Resveratrol works synergistically with vitamin E to stabilized cell mem- branes and prevent lipid peroxida- tion. Finally, resveratrol acts through the Nrf-2 pathway to promote synthe- sis of endogenous antioxidants includ- ing hemoxygenase 1, superoxide dis- mutase and catalase. A recent study has provided some of the first evi- dence that a topical resveratrol formu- lation can activate endogenous antiox- idant production via the Nrf2 pathway in human skin.11 Thus, resveratrol is unique among antioxidants in its ability to boost intrinsic antioxidant capacity. Because of its chemical structure, resveratrol is a member of the stilben- oid group of polyphenols. Like the syn- thetic estrogen diethylstilbesterol, res- veratrol has phytoestrogenic effects and is an estrogen beta receptor agonist.12 It is known that estrogen replacement therapy in post-menopausal women can mitigate at least some of the collagen loss that is synonymous with advancing age. Thus, resveratrol is of interest as an estrogen alternative that can provide the skin benefits of estrogen without the side effects. Stilbene polyphenols can also inhibit tyrosinase and lighten hy- perpigmentation that is common in ac- tinically damaged skin. SKIN AGING AND MITROCHONDRIAL OXIDATIVE STRESS The role of mitochondrial oxidative stress in skin aging has been reviewed by Krut- mann and Schroeder.13 It is known that RESVERATROL see page 34 Scientificstudies havedemonstrated thatresveratrol extendslifespan inavarietyof animalspecies. ES617948_DT0615_010.pgs 05.22.2015 02:34 ADVblackyellowmagentacyan
- 11. Topicort® (desoximetasone cream USP) 0.05% and Topicort® (desoximetasone ointment USP) 0.05% are indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses. Topicort® (desoximetasone cream USP) 0.05% and Topicort® (desoximetasone ointment USP) 0.05% are contraindicated in those patients with a history of hypersensitivity to any of the components of the preparation. Important Safety Information The following local adverse reactions are reported infrequently with topical corticosteroids, but may occur more frequently with the use of occlusive dressings. These reactions are listed in an approximate decreasing order of occurrence: Burning, itching, irritation, dryness, folliculitis, hypertrichosis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, maceration of the skin, secondary infection, skin atrophy, striae, and miliaria. Because of the potential for systemic absorption, use of topical corticosteroids may require that patients be periodically evaluated for HPA axis suppression. Pediatric patients may demonstrate greater susceptibility to topical corticosteroid-induced HPA axis suppression and Cushing’s syndrome than mature patients because of a larger skin surface area to body weight ratio. Hypothalamic-pituitary-adrenal (HPA) axis suppression, Cushing’s syndrome, and intracranial hypertension have been reported in pediatric patients receiving topical corticosteroids. Administration of topical corticosteroids to pediatric patients should be limited to the least amount compatible with an effective therapeutic regimen. Chronic corticosteroid therapy may interfere with the growth and development of pediatric patients. Before prescribing, please see brief Prescribing Information on reverse side. References: 1. Topicort® Cream 0.05% Prescribing Information. Taro Pharmaceuticals U.S.A., Inc. 2. Topicort® Ointment 0.05% Prescribing Information. Taro Pharmaceuticals U.S.A., Inc. 1,2 ® a division of Taro Pharmaceuticals U.S.A., Inc. 3 Skyline Drive, Hawthorne, NY 10532 www.taropharma.com TaroPharma® and Topicort® are registered trademarks of Taro Pharmaceuticals U.S.A., Inc. AD100-0037 © 2015 Taro Pharmaceuticals U.S.A., Inc. May 2015 ES615028_DT0615_011_FP.pgs 05.16.2015 02:44 ADVblackyellowmagentacyan
- 12. Topicort® (Desoximetasone Cream USP) 0.05% Rx only Topicort® (Desoximetasone Ointment USP) 0.05% Rx only Brief Summary of Prescribing Information. For complete prescribing information For topical use only. Not for oral, ophthalmic, or intravaginal use. INDICATIONS AND USAGE Topicort® (desoximetasone cream USP) 0.05% and Topicort® (desoximetasone manifestations of corticosteroid-responsive dermatoses. CONTRAINDICATIONS Topical corticosteroids are contraindicated in those patients with a history of hypersensitivity to any of the components of the preparation. WARNINGS Keep out of reach of children. PRECAUTIONS General Systemic absorption of topical corticosteroids can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for upon withdrawal of the topical corticosteroid. Because of the potential for systemic absorption, use of topical corticosteroids may require that patients be periodically evaluated for HPA axis suppression. Factors that predispose a patient using a topical corticosteroid to HPA axis suppression include the use of more potent steroids, use over large surface areas, use over prolonged periods, use under occlusion, use on an altered skin barrier, and use in patients with liver failure. An ACTH stimulation test may be helpful in evaluating patients for HPA axis suppression. If HPA axis suppression is documented, an attempt should be made to gradually withdraw the drug, to reduce the frequency of application, or require supplemental systemic corticosteroids. Recovery of HPA axis function is generally prompt and complete upon discontinuation of topical corticosteroids. Cushing’s syndrome, hyperglycemia, and unmasking of latent diabetes mellitus can also result from systemic absorption of topical corticosteroids. Use of more than one corticosteroid-containing product at the same time may increase the total systemic corticosteroid exposure. Pediatric patients may be more susceptible to systemic toxicity from use of topical corticosteroids. Local Adverse Reactions with Topical Corticosteroids Local adverse reactions may be more likely to occur with occlusive use, prolonged useoruseofhigherpotencycorticosteroids.Reactionsmayincludeatrophy,striae, telangiectasias, burning, itching, irritation, dryness, folliculitis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, and miliaria. Some local adverse reactions may be irreversible. Allergic Contact Dermatitis with Topical Corticosteroids Allergic contact dermatitis to any component of topical corticosteroids is usually diagnosed by a failure to heal rather than a clinical exacerbation. Clinical Concomitant Skin Infections Concomitant skin infections should be treated with an appropriate antimicrobial agent. If the infection persists, Topicort® (desoximetasone cream USP) 0.05% or Topicort® (desoximetasone ointment USP) 0.05% should be discontinued until the infection has been adequately treated. Information for the Patient Patients using topical corticosteroids should receive the following information and instructions: 1. This medication is to be used as directed by the physician. It is for external use only. Avoid contact with the eyes. 2. Patients should be advised not to use this medication for any disorder other than for which it was prescribed. 3. The treated skin area should not be bandaged or otherwise covered or wrapped as to be occlusive unless directed by the physician. 4. Patients should report any signs of local adverse reactions, especially under occlusive dressings. 5. Other corticosteroid-containing products should not be used with Topicort® (desoximetasone cream USP) 0.05% or Topicort® (desoximetasone ointment As with other corticosteroids, therapy should be discontinued when control is achieved. If no improvement is seen within 4 weeks, contact the physician. Laboratory Tests The following tests may be helpful in evaluating the hypothalamic-pituitary- adrenal (HPA) axis suppression: Urinary free cortisol test ACTH stimulation test Carcinogenesis, Mutagenesis, and Impairment of Fertility Long-term animal studies have not been performed to evaluate the carcinogenic Desoximetasone was nonmutagenic in the Ames test. Corticosteroids have been shown to be teratogenic in laboratory animals when administered systemically at relatively low dosage levels. Some corticosteroids havebeenshowntobeteratogenicafterdermalapplicationinlaboratoryanimals. Desoximetasone has been shown to be teratogenic and embryotoxic in mice, rats, and rabbits when given by subcutaneous or dermal routes of administration in doses 15 to 150 times the human dose of Topicort® (desoximetasone cream USP) 0.05%, or Topicort® (desoximetasone ointment USP) 0.05%. There are no adequate and well-controlled studies in pregnant women on ® (desoximetasone cream USP) 0.05% or Topicort® (desoximetasone ointment the potential risk to the fetus. Drugs of this class should not be used extensively on pregnant patients, in large amounts, or for prolonged periods of time. Nursing Mothers It is not known whether topical administration of corticosteroids could result in Systemicallyadministeredcorticosteroidsaresecretedintobreastmilkinquantities be exercised when topical corticosteroids are administered to a nursing woman. Pediatric Use Pediatric patients may demonstrate greater susceptibility to topical corticosteroid-induced HPA axis suppression and Cushing’s syndrome than mature patients because of a larger skin surface area to body weight ratio. Hypothalamic-pituitary-adrenal (HPA) axis suppression, Cushing’s syndrome, and intracranial hypertension have been reported in pediatric patients receiving topical corticosteroids. Manifestations of adrenal suppression in pediatric patients include linear growth retardation, delayed weight gain, low plasma cortisol levels, and absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema. Administration of topical corticosteroids to pediatric patients should be limited corticosteroid therapy may interfere with the growth and development of pediatric patients. ADVERSE REACTIONS The following local adverse reactions are reported infrequently with topical corticosteroids, but may occur more frequently with the use of occlusive dressings. These reactions are listed in an approximate decreasing order of occurrence: Burning, itching, irritation, dryness, folliculitis, hypertrichosis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, maceration of the skin, secondary infection, skin atrophy, striae, and miliaria. In controlled clinical studies the incidence of adverse reactions were low 0.8% for Topicort® (desoximetasone cream USP) 0.05% and included pruritus, erythema, vesiculation, and burning sensation. The incidence of adverse reactions was low (0.2%) for Topicort® (desoximetasone ointment USP) 0.05% and included mild burning sensation at the site of application. OVERDOSAGE PRECAUTIONS). Mfd. by: Taro Pharmaceuticals Inc., Brampton, Ontario, Canada L6T 1C1 Dist. by: TaroPharma a division of Taro Pharmaceuticals U.S.A., Inc., Hawthorne, NY 10532 Topicort® and TaroPharma® are registered trademarks of Taro Pharmaceuticals FDA. Visitwww.fda.gove/Safery/MedWatch/default.htm, or call 1-800-FDA-1088. Issued: April 2014 ES615015_DT0615_012_FP.pgs 05.16.2015 02:43 ADVblack
- 13. 13JUNE 2015 ⁄ DERMATOLOGYTIMES.COM CLINICAL DERMATOLOGY 14 21GENITAL HERPES PREVENTION Vaccine with deleted glycoprotein shows promising results TATTOOS MAY STOP APPLE WATCH Light-absorbing quality of tattoo ink keeps popular device from functioning properly A is the leading derma- tologic diagnosis among the popula- tionofAmericanscollectivelyreferred to as people with skin of color: Afri- canAmericans,Hispanics/Latinosand Asians/PacificIslanders.1 Dermatolo- gist Andrew F. Alexis, M.D., M.P.H., who re- ported that finding in the June 2014 Journal of Drugs in Dermatol- ogy, also conducted a comparativestudyathis Skin of Color Center in NewYorkCityandfoundthatacnewas theleadingdiagnosisinAfricanAmer- icans, Hispanic/Latinos and Cauca- sians.2 Skin of color patients exhibit clinical and therapeutic nuances that affect acne management. Dermatol- ogists should understand how these patients present, clinically; how to treatthemsafely;culturalfactors;and their specific desired treatment end- points. Dr. Alexis, who is dermatol- ogychairatMountSinaiSt.Lukesand MountSinaiRoosevelt,NewYork,N.Y., presentedonacneandrosacea inskin of color during the May 2015 Skin of Color Seminar in New York City. WHAT DERMATOLOGISTS SHOULD KNOW These are distinct features of acne in patients with skin of color, according toDr.Alexis:skinofcoloracnepatients haveatendencytodeveloppostinflam- matory hyperpigmentation, which is frequently of equal or greater concern to the patient as the acne itself. They have a higher risk than Caucasian pa- tients of keloids or hypertrophic scars arising at sites of moderate-to-severe acne.Thescarsareespeciallylikelyon the chest and back. Cultural practices in patients with skin of color may in- crease acne risk. Many African Amer- icans, for example, use hair products that contain oils, which can be come- dogenic and produce “pomade acne,” he says. Another example: the use of cocoabutteriscommonpracticeamong African Americans, who hope to im- prove their complexions. “[There is a] widely held but unproven belief that cocoa butter evens the skin tone,” Dr. Alexis says. “This can, in turn, exac- erbate acne because of its comedog- enic nature.” LATEST RESEARCH FINDINGS Because of higher risks of pigmentary abnormalitiesamongpatientswithskin of color, dermatologists should eval- uate the safety of topical acne prod- ucts that might irritate the skin before “With the SGR charade behind us, physicians have an added measure of practice stability that will enable us to more closely focus on meaningful physician payment and delivery reforms that facilitate access to high quality patient care... Dermatology patients can also breathe a little easier today, knowing that the bill also alleviates additional co-pays for surgical or procedural follow- up care. It was unconscionable that under a previ- ously established policy, patients would have to open their wallets to simply have their stitches out. Not only is this bill good policy, it’s the right thing for patients.” Mark G. Lebwohl, M.D., AAD President SOURCE: HTTP://BIT.LY/PAYMENTFORMULA ACNE IN SKIN OF COLOR see page 14 Acne in skin of color LISETTE HILTON | STAFF CORRESPONDENT It turns out, the more you debride, the better... If you had a sort of molecu- lar scalpel, you could debride right to that edge and get to the tissue that’s capable of healing.” Robert S. Kirsner, M.D., Ph.D. MauiDerm 2015 Wound Healing See story page 1 QuotableDTExtra Dr. Alexis Keloid scarring on the chest Photo: Andrew F. Alexis, M.D. ES615967_DT0615_013.pgs 05.20.2015 00:45 ADVblackyellowmagentacyan
- 14. ® JUNE 2015 ⁄ DERMATOLOGYTIMES.COM 14 CLINICAL DERMATOLOGY recommending patients use them, Dr. Alexissays.“Fortunately,therehavebeen studiesofthemajortopicalagentsthat have specifically looked at tolerability andoverallsafetyinpatientswithhigher Fitzpatricktypes.Theseincluderecent studiesofadapalene0.1%-benzoylper- oxide 2.5% gel in black patients3 … and dapsonegel,5%inadultskinofcolorfe- males with facial acne vulgaris, which waspresentedattheSkinofColorSem- inar Series annual meeting,” he says. TREATING SKIN OF COLOR ACNE PATIENTS Controlling inflammation associated with acne is paramount in skin of color patients, given the higher risk for long lasting sequelae, such as postinflam- matory hyperpigmentation and, in se- vere cases involving the trunk, keloids, Dr.Alexissays.“Thiscanbeachievedby carefulselectionofwell-toleratedandef- fectiveagentsthathaveanti-inflamma- tory effects relevant to acne. These in- clude topical retinoids [especially ada- palene],topicaldapsoneand,indirectly, topicalbenzoylperoxide,whichinhibits P. acnes — an important driver of in- flammationinacne,”hesays.“Italsoin- cludes oral tetracyclines,” doxycycline and minocycline, in particular. BEST PRACTICES Itmightbeintuitivefordermatologists toundertreatskinofcoloracnepatients because of the risks of hyperpigmen- tation and scarring. But that’s not the right approach. In fact, Dr. Alexis says, undertreatingskinofcolorpatientswith acnecouldleadtomoreseverepostin- flammatoryhyperpigmentation.And,in thecaseofmoderate-to-severetruncal acne,undertreatmentcouldincreasethe riskofhypertrophicorkeloidscarring. “Carefully select efficacious, but well tolerated topical regimens such that ir- ritationisavoided[sincethiscaninduce pigment alterations]. Examples of well- tolerated agents are those with well-for- mulated vehicles, such as aqueous gels and creams [as opposed to ethanolic gels, which are more drying and poten- tiallyirritating],”hesays.“Startingretin- oidsatalowerconcentration,thenwork- ing upward over time is a good strategy toensuretolerability.Usingbenzoylper- oxideatconcentrationsof2.5-5%ispre- ferred over higher concentrations. Mi- cronized or micro-dispersed formula- tions are also better tolerated.” DT Disclosure: Dr. Alexis is an investigator for Allergan and a consultant or advisory board member with Allergan, Bayer, Galderma and Valeant. References: 1. Alexis AF. Acne vulgaris in skin of color: under- standing nuances and optimizing treatment out- comes. J Drugs Dermatol. 2014 Jun;13(6):s61-5. 2. Callender VD, Alexis AF, Daniels SR, Kawata AK, Burk CT, Wilcox TK, Taylor SC. Racial differences in clinical characteristics, perceptions and behaviors, and psychosocial impact of adult female acne. J Clin Aesthet Dermatol. 2014 Jul;7(7):19-31. 3. Alexis AF, Johnson LA, Kerrouche N, Callender VD. A subgroup analysis to evaluate the efficacy and safety of adapalene-benzoyl peroxide topical gel in black subjects with moderate acne. J Drugs Der- matol. 2014 Feb;13(2):170-4. ACNE IN SKIN OF COLOR: Clinical and therapeutic nuances from page 13 Acne with postinflammatory hyperpigmentation Photo: Andrew F. Alexis, M.D. Controlling inflammation associated with acne is paramount in skin of color patients. P toconvincetheirteen- ager not to get a tattoo might try this: GivetheteenanAppleWatchandpass along the following news. According to a CNN report, some Apple Watch wearers say their wrist tattoos prevent the device’s heart-rate sensorfromfunctioningproperly.And since the Apple Watch uses the wear- er’s heart rate to determine whether thedeviceisbeingworn,awearerwho has wrist tattoos might not be able to placecalls,receivenotificationsoruse certain apps. TattooedAppleWatchwearershave complained about the issue on social media, and Apple blog iMore con- firmed that the problem does indeed exist. AccordingtoCNN,thewayinwhich the Apple Watch senses the wearer’s heartbeat causes the problem. Apple says the back of the device flashes green and infrared light at the wear- er’s skin, and the light is absorbed or reflectedbytheblood.Whentheheart pumps more blood to the wrist area, the device senses it, and it also senses the diminished amount of blood be- tween beats. The Apple Watch calcu- latesthewearer’sheartratebysensing the timing between heartbeats. Solid-colored tattoos — especially red ones — also absorb the green light and reflect the red light. Black tattoos, which absorb both green and red light, can also interfere with the AppleWatch’sheart-ratesensor.Dark- coloredskin,scarsandskinabrasions, which are translucent, don’t interfere withthedevice’sheart-ratesensor.Tat- too ink is opaque, however, and pre- vents light from penetrating the skin. CNN reports that users have found thatturningofftheAppleWatch’swrist detection function allows notifica- tions to come in, but it also prevents the wearer from using the Apple Pay function and receiving calls. DT Tattoos may stop Apple Watch BILL GILLETTE | STAFF CORRESPONDENT ES615966_DT0615_014.pgs 05.20.2015 00:45 ADVblackyellowmagentacyan
- 15. Unilever is a pioneer in creating great products and brands. What truly sets us apart is that we have spent decades cultivating our expertise in caring for the stratum corneum (SC)—from scalp to toe—and, at the same time, designing products that millions of people truly enjoy using every day. With over 20,000 patents and patent applications, Unilever has always been at the forefront of scientific breakthroughs—from the discovery of the filaggrin protein and its role in creating natural moisturizing factor (NMF), to the novel combination of ultra-mild surfactants like Directly Esterified Fatty Isethionate (DEFI) and glycinate, that help preserve both SC proteins and lipids during cleansing—all while exceeding consumer expectations. Our journey continues, with a deep commitment to measuring product effects across the entire SC, helping rebuild skin with brands and innovations you and your patients will love. Science that leads the way. Products that capture the heart. © 2015 Unilever 810899 Dove is a Registered Trademark ES621757_DT0615_015_FP.pgs 05.27.2015 02:19 ADVblackyellowmagentacyan
- 16. ® JUNE 2015 ⁄ DERMATOLOGYTIMES.COM 16 CLINICAL DERMATOLOGY threetosixtimesasmanywoundsthat we must repair. So we should know how to take care of those wounds.” To that end, Dr. Kirsner suggests viewing wounds along a continuum of healing outcomes. Between nor- mal healing and chronic wounds lies a wide spectrum of abnormal healing. TheHolyGrail,hesays,isscarlessheal- ing,asoccursinfetusesduringthefirst two trimesters of gestation. STIFLING STRESS REACTIONS Indirectly,stressorssuchasanxiety,isola- tionandevenpostoperativepaincanre- ducewound-healingratesupto40%.1 Such stressors release steroids, which stimu- latethepituitarytoreleaseglucocorticoid hormones.Thesehormonesdiminishthe amountofpro-healingcytokines,suchas interleukin (IL)-1, vascular endothelial growth factor (VEGF) and transforma- tive growth factor beta (TGFb), availa- bletoreachthewound,Dr.Kirsnersays. Conversely, he says, physical and mental exercise, a positive attitude, social interaction and pain reduction all reduce stress, mediated by oxy- tocin. “Someday perhaps we’ll be giv- ing oxytocin to reduce stress and im- prove healing.” For now, “consider cre- ating a stress-free environment for our patients.” This can include controlling operative and postoperative pain, he says, and perhaps postponing elective proceduresifpatientshaverecentlyex- perienced stressful life events. Like steroids, catecholamines re- leased during stress also delay healing. Dr. Kirsner says that the success of sys- temicpropranololinreducingchildhood hemangiomas2 fueledthediscoverythat beta blockers block catecholamines — a mechanism that’s proving useful in manyindications.Forinstance, inaran- domized, controlled trial with 79 burn patients, those who received a systemic beta blocker healed faster (16.13 ± 7.4 days versus 21.52 ± 7.94 days, P = 0.004) than control-group patients.3 The first use of beta blockers in heal- ing chronic wounds involved a 43-year- old female with a persistent open surgi- cal wound after her second heart trans- plant.4 Withtopicaltimolol0.5%,saysDr. Kirsner,whotreatedher,thewoundepi- thelialized in about 8 weeks. More recently, he published a case series in which three of five diverse chronic wounds treated with topical timololhealedcompletely,andafourth achieveddramaticimprovement.5 “Not everybody does well. But beta blockers provide an opportunity to reverse pre- viously nonhealing wounds.” Clinically, Dr. Kirsner uses topical timolol for modest superficial wounds, toachievenotgranulationbutepitheli- alization. Since optimal dosing has not yet been determined, he adds, “We use itconsistentwiththestandardofcare,” which ranges from daily to weekly. SKIN GRAFTING TOOLS Forskingrafting,theCelluTomeEpider- mal Harvesting System (Kinetic Con- cepts Inc./Acelity), commercially avail- able in the United States and Europe, allows harvesting of epidermal grafts without creating donor-site scars. “You placethislittledevice,withoutanesthe- sia,onthedonorsite(typicallytheinner thigh) for 20 to 45 minutes.” Using suc- tion, the device raises approximately 30 tinymicrodomesofepidermis.Pressinga levershearsallthegraftsoff,sotheycan be placed on a wound-dressing sheet. “Then you transfer those little pieces of epidermis onto the wound. When you putthemincultureonpatients’skin,the keratinocytes migrate out of the micro- domes” to speed wound healing.6 Specifically, says Dr. Kirsner, the mi- crografts deliver growth factors to the wound, resulting in edge advance- ment. Confocal microscopy moreover has shown that the grafts indeed “take” wherethey’replanted,resultingindotsof newepidermisacrossthewoundsurface, he says. Because the process also trans- fers melanocytes, it works for vitiligo as well.Atthedonorsite,“youcan’teventell anything was done 2 days later.” The fact that the CelluTome creates verylittledonor-siteinflammationalso makes it helpful in addressing wounds left by pyoderma gangrenosum, after appropriatepharmaceuticaltreatment,7 and perhaps in other conditions where inflammationcontraindicatesconven- tional skin grafting, he says. Becausetheprocedurecreatesmin- imal donor-site disruption and is easy to perform, Dr. Kirsner has used it in otherindications,includingsicklecell ulcers and nonhealing wounds in an elderly male treated for several squa- mous cell carcinomas. Conversely, the Xpansion Micro- Autografting Kit (SteadMed/Applied Tissue Technologies) minces harvested skin to create 0.8 mm x 0.8 mm split- WOUND HEALING see page 18 New wound-healing tools arising from evolving knowledge of the wound-healing process include drugs, debridement techniques, advanced skin grafting tools and stem cell delivery strategies. QUICK READ WOUND HEALING: Diverse tools and technique advances from page 1 Day 1 Week 5 Month 9 28-year-old female with sickle cell ulcer before and after treatment with CelluTome Epidermal Harvesting System Photo credit: Michael Kirsner, M.D. ES618074_DT0615_016.pgs 05.22.2015 21:14 ADVblackyellowmagentacyan
- 17. * Head-to-head comparison of Topical Hydrocortisone 1%, Topical Diphenhydramine 2% and TriCalm® in a cowhage-induced itch model. Data on file. BETTER SCIENCE. BETTER ITCH RELIEF. BETTER SCIENCE. BETTER ITCH RELIEF. TriCalm® is 5 times more effective at reducing itch than hydrocortisone 1% and 6 times more effective than diphenhydramine 2%.* ! ! Request FREE samples at www.tricalm.com/hcps Reduction of Total Itch Perceived (AUC) ES615031_DT0615_017_FP.pgs 05.16.2015 02:44 ADVblackyellowmagentacyan
- 18. ® JUNE 2015 ⁄ DERMATOLOGYTIMES.COM 18 CLINICAL DERMATOLOGY thickness skin grafts that can cover an area 100 times the size of the original graft. There’s no need to orient the mi- crografts dermal side down, says Dr. Kirsner.“Thosecells...flipover,ontheir own,” boosting the wound’s healing ca- pability. Somewhat similarly, he says, using hair-transplantation techniques pro- vides better stimulation of wound heal- ing than traditional pinch biopsy tech- niques, perhaps because hair follicles contain factors such as stem cells. Pres- ently,Dr.Kirsnerandhiscolleaguesare studying the interface between hair fol- licles and wound healing. “We believe thattheysharemanyofthesamemecha- nisms,andsomeoftheinformationand hairbiologythatwe’velearnedfromhair diseases can be applied to wounds.” To improve the quality of conven- tional skin grafts, Dr. Kirsner adds, “pre-wounding” donor-area skin ap- pears helpful.8 In two patients with chroniclegulceration,“Wetookpinch biopsies, creating wounds, but didn’t harvest the skin. We covered it for 3 days, which turned on the healing process in the grafts, then transferred them onto wounds.” Pre-wounded grafts achieved marked improvement inulcer-bedgranulationtissue,andin- creased edge advancement. DEBRIDEMENT: GO BIG Although debridement represents the standard of care for chronic wounds, Dr. Kirsner says, wound-healing spe- cialists have long debated how exten- sivelyoneshoulddebride.Basedonase- riesof312,774chronicwounds,“Itturns out,themoreyoudebride,thebetter.”9 Biopsies have shown that debride- ment removes key factors — includ- ing c-Myc and nuclear expression of beta-catenin — that prevent keratino- cytesfrommigratingoutwardfromthe edges of chronic wounds.10 “Therefore,youwanttonotonlyde- bride the base of the wound, but also the edge,” to reach healthier skin.11 In fact, “If you had a sort of molecu- lar scalpel, you could debride right to thatedgeandgettothetissuethat’sca- pable of healing. So we propose envi- sioningdebridementalmostlikeMohs surgery,” wherein one examines suc- cessive layers of excised tissues not for cancer,butforc-Mycandbeta-catenin. DELIVERING STEM CELLS In chronic wounds such as diabetic ul- cers, Dr. Kirsner says, the pathway by whichfactorsreleasedfromthewound traveltothebonemarrowandstimulate progenitorcells,causingstemcellstomi- grate to the wound, is perturbed. How- ever,headds,casereportsshowthatap- plyingbonemarrow-derivedstemcells directlyintoafreshlydebridedwound— viaspray,injectionordressings—speeds healing while minimizing scarring.12 Similarly,oneofthefewrandomized, controlled trials in this area showed that placing MSCs into 18 diabetic ul- cers led to statistically significant im- provement versus placebo.13 As such, “There’s a lot of potential here, using stem cells including bone marrow-de- rived stem cells to speed healing, and work is ongoing.” MSCs show particular potential be- cause they’re both anti-inflammatory and antifibrotic, Dr. Kirsner says. In one case report, injecting MSCs into a severe radiation burn at the time of surgery significantly improved scar- ring.14 A more recent study revealed howMSCsdothis,hesays.Onceplaced intoawound,heexplains,theyquickly die, releasing factors that improve scarring.15 “Work is underway to fig- ure out exactly what those factors are. Ifwecanidentifythem,maybewedon’t even need the MSCs.” Presently, Dr. Kirsner’s colleagues are evaluating mechanisms for deliver- ingMSCs.Inthisregard,hesaysthatla- ser-created channels appear more effi- cientthandressings,spraysorinjections, particularly for burns and war wounds. SIMPLE SCAR REDUCTION Forfull-thicknesssurgicalwounds,“The standardofcareistosutureitclosed.But often,wesutureitclosed,maybeapplya dressing,andbelieveourworkisdone.” Even with optimal surgical technique, however,countertensionmaywidenthe scar over time. To address this problem, Dr. Kirsner suggested considering Embrace Ad- vanced Scar Therapy (Neodyne Bio- sciences), a commercially available dressing that is applied weekly for up to eight weeks, starting 1 week after sur- gery. “It’s a simple tool to reduce post- surgical skin tension, which results in better scar formation.” Injecting botu- linum toxin type A may fulfill the same function,saysDr.Kirsner.Animalstud- iessuggestthisstrategyworks,16 “Butthe human studies are not yet robust.” DT Disclosure: Dr. Kirsner is an advisor for Acelity. REFERENCES 1.Marucha PT, Kiecolt-Glaser JK, Favagehi M. Mu- cosal wound healing is impaired by exami- nation stress. Psychosom Med. 1998 May- Jun;60(3):362-5. 2.Léauté-Labrèze C, Dumas de la Roque E, Hubi- che T, Boralevi F, Thambo JB, Taïeb A. Proprano- lol for severe hemangiomas of infancy. N Engl J Med. 2008 Jun 12;358(24):2649-51. doi: 10.1056/ NEJMc0708819. 3.Mohammadi AA, Bakhshaeekia A, Alibeigi P, et al. Efficacy of propranolol in wound heal- ing for hospitalized burn patients. J Burn Care Res. 2009 Nov-Dec;30(6):1013-7. doi: 10.1097/ BCR.0b013e3181b48600. 4.Tang JC, Dosal J, Kirsner RS. For topical timo- lol for a refractory wound. Dermatol Surg. 2012 Jan;38(1):135-8. WOUND HEALING: Diverse tools and technique advances from page 16 “We create three to six times as many wounds that we must repair [versus general surgeons and plastic surgeons]. So we should know how to take care of those wounds.” Robert S. Kirsner, M.D., Ph.D. Miami Fla. WOUND HEALING see page 21 ES618078_DT0615_018.pgs 05.22.2015 21:14 ADVblackyellowmagentacyan
- 20. BRIEF SUMMARY OF FULL PRESCRIBING INFORMATION This Brief Summary does not include all the information needed to use ONEXTON Gel safely and effectively. See full prescribing information for ONEXTON Gel. ONEXTON™ (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/3.75%, for topical use Initial U.S. Approval: 2000 CONTRAINDICATIONS Hypersensitivity ONEXTON Gel is contraindicated in those individuals who have shown hypersensitivity to clindamycin, benzoyl peroxide, any components of the formulation, or lincomycin. Anaphylaxis, as well as allergic reactions leading to hospitalization, has been reported in postmarketing use with ONEXTON Gel [see Adverse Reactions] WARNINGS AND PRECAUTIONS Colitis/Enteritis Systemic absorption of clindamycin has been demonstrated following topical use of clindamycin. Diarrhea, bloody diarrhea, and colitis (including pseudomembranous colitis) have been reported with the use of topical and systemic clindamycin. If significant diarrhea occurs, ONEXTON Gel should be discontinued. Severe colitis has occurred following oral and parenteral administration of clindamycin with an onset of up to several weeks following cessation of therapy. Antiperistaltic agents such as opiates and diphenoxylate with atropine may prolong and/or worsen severe colitis. Severe colitis may result in death. Studies indicate toxin(s) produced by Clostridia is one primary cause of antibiotic-associated colitis. The colitis is usually characterized by severe persistent diarrhea and severe abdominal cramps and may be associated with the passage of blood and mucus. Stool cultures for Clostridium difficile and stool assay for C. difficile toxin may be helpful diagnostically. Ultraviolet Light and Environmental Exposure Minimize sun exposure (including use of tanning beds or sun lamps) following drug application [see Nonclinical Toxicology]. ADVERSE REACTIONS The following adverse reaction is described in more detail in the Warnings and Precautions section of the label: Colitis [see Warnings and Precautions]. Clinical Trials Experience Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates observed in the clinical trials of another drug and may not reflect the rates observed in clinical practice. These adverse reactions occurred in less than 0.5% of subjects treated with ONEXTON Gel: burning sensation (0.4%); contact dermatitis (0.4%); pruritus (0.4%); and rash (0.4%). During the clinical trial, subjects were assessed for local cutaneous signs and symptoms of erythema, scaling, itching, burning and stinging. Most local skin reactions either were the same as baseline or increased and peaked around week 4 and were near or improved from baseline levels by week 12. The percentage of subjects that had symptoms present before treatment (at baseline), during treatment, and the percent with symptoms present at week 12 are shown in Table 1. Table 1: Local Skin Reactions - Percent of Subjects with Symptoms Present. Results from the Phase 3 Trial of ONEXTON Gel 1.2%/3.75% (N = 243) *Mod. = Moderate Postmarketing Experience Because postmarketing adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Anaphylaxis, as well as allergic reactions leading to hospitalizations, has been reported in postmarketing use of products containing clindamycin phosphate/benzoyl peroxide. DRUG INTERACTIONS Erythromycin Avoid using ONEXTON Gel in combination with topical or oral erythromycin- containing products due to its clindamycin component. In vitro studies have shown antagonism between erythromycin and clindamycin. The clinical significance of this in vitro antagonism is not known. Concomitant Topical Medications Concomitant topical acne therapy should be used with caution since a possible cumulative irritancy effect may occur, especially with the use of peeling, desquamating, or abrasive agents. If irritancy or dermatitis occurs, reduce frequency of application or temporarily interrupt treatment and resume once the irritation subsides. Treatment should be discontinued if the irritation persists. Neuromuscular Blocking Agents Clindamycin has been shown to have neuromuscular blocking properties that may enhance the action of other neuromuscular blocking agents. ONEXTON Gel should be used with caution in patients receiving such agents. USE IN SPECIFIC POPULATIONS Pregnancy Pregnancy Category C. There are no adequate and well-controlled studies in pregnant women treated with ONEXTON Gel. ONEXTON Gel should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Animal reproductive/developmental toxicity studies have not been conducted with ONEXTON Gel or benzoyl peroxide. Developmental toxicity studies of clindamycin performed in rats and mice using oral doses of up to 600 mg/kg/day (240 and 120 times amount of clindamycin in the highest recommended adult human dose based on mg/m2 , respectively) or subcutaneous doses of up to 200 mg/kg/day (80 and 40 times the amount of clindamycin in the highest recommended adult human dose based on mg/m2 , respectively) revealed no evidence of teratogenicity. Nursing Mothers It is not known whether clindamycin is excreted in human milk after topical application of ONEXTON Gel. However, orally and parenterally administered clindamycin has been reported to appear in breast milk. Because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to use ONEXTON Gel while nursing, taking into account the importance of the drug to the mother. Pediatric Use Safety and effectiveness of ONEXTON Gel in pediatric patients under the age of 12 have not been evaluated. Geriatric Use Clinical trials of ONEXTON Gel did not include sufficient numbers of subjects aged 65 and older to determine whether they respond differently from younger subjects. NONCLINICAL TOXICOLOGY Carcinogenesis, Mutagenesis, Impairment of Fertility Carcinogenicity, mutagenicity and impairment of fertility testing of ONEXTON Gel have not been performed. Benzoyl peroxide has been shown to be a tumor promoter and progression agent in a number of animal studies. Benzoyl peroxide in acetone at doses of 5 and 10 mg administered topically twice per week for 20 weeks induced skin tumors in transgenic Tg.AC mice. The clinical significance of this is unknown. Carcinogenicity studies have been conducted with a gel formulation containing 1% clindamycin and 5% benzoyl peroxide. In a 2-year dermal carcinogenicity study in mice, treatment with the gel formulation at doses of 900, 2700, and 15000 mg/kg/day (1.8, 5.4, and 30 times amount of clindamycin and 2.4, 7.2, and 40 times amount of benzoyl peroxide in the highest recommended adult human dose of 2.5 g ONEXTON Gel based on mg/m2 , respectively) did not cause any increase in tumors. However, topical treatment with a different gel formulation containing 1% clindamycin and 5% benzoyl peroxide at doses of 100, 500, and 2000 mg/kg/day caused a dose-dependent increase in the incidence of keratoacanthoma at the treated skin site of male rats in a 2-year dermal carcinogenicity study in rats. In an oral (gavage) carcinogenicity study in rats, treatment with the gel formulation at doses of 300, 900 and 3000 mg/kg/day (1.2, 3.6, and 12 times amount of clindamycin and 1.6, 4.8, and 16 times amount of benzoyl peroxide in the highest recommended adult human dose of 2.5 g ONEXTON Gel based on mg/ m2 , respectively) for up to 97 weeks did not cause any increase in tumors. In a 52-week dermal photocarcinogenicity study in hairless mice, (40 weeks of treatment followed by 12 weeks of observation), the median time to onset of skin tumor formation decreased and the number of tumors per mouse increased relative to controls following chronic concurrent topical administration of the higher concentration benzoyl peroxide formulation (5000 and 10000 mg/kg/day, 5 days/week) and exposure to ultraviolet radiation. Clindamycin phosphate was not genotoxic in the human lymphocyte chromosome aberration assay. Benzoyl peroxide has been found to cause DNA strand breaks in a variety of mammalian cell types, to be mutagenic in S. typhimurium tests by some but not all investigators, and to cause sister chromatid exchanges in Chinese hamster ovary cells. Fertility studies have not been performed with ONEXTON Gel or benzoyl peroxide, but fertility and mating ability have been studied with clindamycin. Fertility studies in rats treated orally with up to 300 mg/kg/day of clindamycin (approximately 120 times the amount of clindamycin in the highest recommended adult human dose of 2.5 g ONEXTON Gel, based on mg/m2 ) revealed no effects on fertility or mating ability. PATIENT COUNSELING INFORMATION See FDA-approved patient labeling (Patient Information). Distributed by: Valeant Pharmaceuticals North America LLC, Bridgewater, NJ 08807 Manufactured by: Contract Pharmaceuticals Limited Mississauga, Ontario, Canada L5N 6L6 U.S. Patents 5,733,886 and 8,288,434 Issued 11/2014 9389300 DM/ONX/14/0031(1) Before Treatment (Baseline) Maximum During Treatment End of Treatment (Week 12) Mild Mod.* Severe Mild Mod.* Severe Mild Mod.* Severe Erythema 20 6 0 28 5 <1 15 2 0 Scaling 10 1 0 19 3 0 10 <1 0 Itching 14 3 <1 15 3 0 7 2 0 Burning 5 <1 <1 7 1 <1 3 <1 0 Stinging 5 <1 0 7 0 <1 3 0 <1 ES615029_DT0615_020_FP.pgs 05.16.2015 02:44 ADVblack
- 21. 21JUNE 2015 ⁄ DERMATOLOGYTIMES.COM CLINICAL DERMATOLOGY 5.Braun LR, Lamel SA, Richmond NA, Kirsner RS. Topical timolol for recalcitrant wounds. JAMA Der- matol. 2013 Dec;149(12):1400-2. doi: 10.1001/jama- dermatol.2013.7135. 6.Osborne SN, Schmidt MA, Derrick KL, Harper JR. Epidermal micrografts produced via an automated and minimally invasive tool form at the dermal-epi- dermal junction and contain proliferative cells that secrete wound-healing growth factors. Adv Skin Wound Care. (In press). 7.Richmond NA, Jamel SA, Braun LR, Vivas AC, Ser- ena T, Kirsner RS. Epidermal grafting using a novel suction blister harvesting system for the treatment of pyoderma gangrenosum. JAMA Dermatol. Pub- lished online August 6, 2014. Accessed February 23, 2015. 8.Kirsner RS, Falanga V, Kerdel FA, Katz MH, Eagl- stein WH. Skin grafts as pharmacological agents: pre-wounding of the donor site. Br J Dermatol. 1996 Aug;135(2):292-6. 9.Wilcox JR, Carter MJ, Covington S. Frequency of debridements and time to heal: a retrospective co- hort study of 312,744 wounds. JAMA Dermatol. 2013 Sep;149(9):1050-8. doi: 10.1001/jamaderma- tol.2013.4960. 10. Brem H, Stojadinovic O, Diegelmann RF, et al. Mo- lecular markers in patients with chronic wounds to guide surgical debridement. Mol Med. 2007 Jan-Feb;13(1-2):30-9. 11. Tomic-Canic M, Ayello EA, Stojadinovic O, Golinko MS, Brem H. Using gene transcription patterns (bar coding scans) to guide wound debride- ment and healing. Adv Skin Wound Care. 2008 Oct;21(10):487-92; quiz 493-4. doi: 10.1097/01. ASW.0000323563.59885.1c. 12. Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 2003 Apr;139(4):510-6. 13. Dash NR, Dash SN, Routray P, Mohapatra S, Mo- hapatra PC. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Re- juvenation Res. 2009 Oct;12(5):359-66. doi: 10.1089/rej.2009.0872. 14. Bey E, Prat M, Duhamel P, et al. Emerging ther- apy for improving wound repair of severe radia- tion burns using local bone marrow-derived stem cell administrations. Wound Repair Regen. 2010. 18:50-58. doi: 10.1111/j.1524-475X.2009.00562.x. 15. Liu S, Jiang L, Li H, et al. Mesenchymal Stem Cells Prevent Hypertrophic Scar Formation via In- flammatory Regulation when Undergoing Apop- tosis. J Invest Dermatol. 2014. 134: 2648–2657. doi:10.1038/jid.2014.169. Published online May 29, 2014. 16. Lee BJ, Jeong JH, Wang SG, Lee JC, Goh EK, Kim HW. Effect of botulinum toxin type A on a rat surgical wound model. Clin Exp Otorhi- nolaryngol. 2009 Mar;2(1):20-7. doi: 10.3342/ ceo.2009.2.1.20. Epub 2009 Mar 26. WOUND HEALING: Diverse tools and technique advances from page 18 T thinking is that an effective HSV-2 vaccine must stimulate thebodytoproduceneutralizingantibod- ies,particularlyagainsttheviralprotein glycoprotein D (gD-2) through which HSV-2 accesses human cells. Research- ersinthepasthavefocusedon“sub-unit” herpesvaccinesthatrelyprimarilyongD-2 astheantigentostimulatethebody’san- tibodyresponse.Noneofthese,however, haspreventedHSV-2infectioninhumans. Using a non-traditional approach, a researchteamattheAlbertEinsteinCol- lege of Medicine, Bronx, N.Y. — headed by Betsy Herold, M.D., and William Ja- cobs Jr., Ph.D. — developed a vaccine that prevented active and latent infec- tions caused by herpes simplex virus type 2 (HSV-2) from occurring in mice and published their findings.1 AccordingtoDr.Jacobs,professor and Leo and Julia Forchheimer chair in mi- crobiologyandimmunologyatEinstein, the team took an approach that runs countertowhatmostscientiststake.“We tookacompletelydifferentapproachand deleted glycoprotein D from the virus,” Dr. Herold, vice chair for research at Einstein, tells Dermatology Times. “The deleted strain, which is markedly attenuated and cannot spread, is com- pletely safe and provided 100% protec- tionagainstbothHSV-1andHSV-2,and prevented the establishment of latency in different mouse models, including a model of skin infection.” Whenthevaccine,calleddelta-gD-2, was given to mice, it provided complete protection against subsequent infec- tion with normal HSV-2. No virus was detected in vaginal or skin tissue of vaccinated mice or in neural tissue, where HSV-2 often lays latent. When unvaccinated mice were challenged with HSV-2, all showed evidence of the virus in the three tissue sites, and all succumbed to the disease. Initial tests suggest that the vaccine is also effective against HSV-1, though further evalua- tion is needed to confirm this. The new vaccine also appears to be safe. The researchers calculated the number of viruses needed to kill mice, then administered 1,000 times that number of delta-g D-2 viruses to mice that lacked immune systems. The result: The mice survived and didn’t develop herpes. “For the first time in history, we have successfully developed a vaccine that prevents HSV-1 and HSV-2, using an approach that experts thought was doomed to fail,” Dr. Jacobs says. “If this vaccineprotectshumansasitdoesmice, herpes could be eradicated.” TheEinsteinteamplanstobeginclin- icaltrialsonhumanswithinafewyears. “We hope that this vaccine can- didate, which elicits a different type of immune response than prior vac- cines, will prove equally protective in cl i n ica l st ud ies,” Dr. Herold says. “We are planning to con- duct t he pre-clin ica l st ud ies required by the Food and Drug Administrationtoallowustomovethis vaccineintoaphase1clinicaltrial.”DT Disclosures: The Albert Einstein College of Medicine has filed patent applications related to this research and is seeking licensing partners able to further de- velop and commercialize this technology. REFERENCES: 1.Petro C, González PA, Cheshenko N, et al. Her- pes simplex type 2 virus deleted in glycoprotein D protects against vaginal, skin and neural disease. Elife. 2015;4 Vaccine has potential to prevent genital herpes BILL GILLETTE | STAFF CORRESPONDENT ES618075_DT0615_021.pgs 05.22.2015 21:14 ADVblackyellowmagentacyan
- 22. ® JUNE 2015 ⁄ DERMATOLOGYTIMES.COM COSMETIC DERMATOLOGY22 29LIPO-202 Fat reduction injectable enters phase 3 trials N – Although pico- second lasers have changed the tat- too-removalgame,oldertechnologies suchasQ-switchedlasersremainvery valuable players, experts say. Nowavailableinthreewavelengths, picosecond lasers have made some of the toughest-to-treat tattoo colors the easiest, says Roy Geronemus, M.D., director of the Laser & Skin Surgery Center of New York and clinical pro- fessor of dermatology at New York University Medical Center. HOME RUN First on the market was the PicoSure (Cynosure),whichoperatesatthe755nm alexandrite wavelength. “Although it can be used for many colors, the home runwiththatdevicehasbeenwithblue Laser tattoo removal: rethinking ink JOHN JESITUS | STAFF CORRESPONDENT There’s a total epidemic of people wanting their tattoos removed.” Eric F. Bernstein, M.D. Ardmore, Penn. Tattoo removal: a booming business See story page 26 New picosecond lasers allow for faster treatment of several once-difficult tattoo inks such as green, blue, red, orange and yellow. Meanwhile, Q-switched laser regimens continue to evolve, sometimes in combination with fractional ablation. QUICK READ Quotable DTExtra Cosmetic tattoo before and after three treatments with a Q-switched Nd:YAG laser Photos: George Hruza, M.D. and green ink.” In previously untreated tattoos,hesays,thislaseroftenremoves these colors in one to three sessions, versus10to15ormorewithQ-switched lasers,whichcouldstillleaveresidualink. Darker colors and resistant tattoos alsofarebetterwiththe755nmpicosec- ondlaserthantheydidwithQ-switched lasers, he adds. In February 2015, the FDA cleared a 532 nm Laser Delivery System that’s available as an add-on to the PicoSure platform. With the 532 nm wavelength, yellow ink—whichpreviouslyprovedverydif- ficult to remove — disappears in one to four treatments.1 “We’re seeing good resultswithredandorangeaswell,”says Dr. Geronemus. Picosecond lasers that combine the 532 nm (KTP) and 1,064 nm Nd:YAG wavelengths include the PicoWay (Syneron & Candela) and the enlighten (Cutera). Eric F. Bernstein, M.D., M.S.E., who was first to use the PicoWay laser, says, “Ifyoucanonlyhaveonelaserfortattoo removal,it’sgottobeanNd:YAGbecause epidermal melanin absorbs less energy atthiswavelengthsoitallowsyoutotreat all skin types. And Nd:YAG lasers are great for removing black ink.” Though less effective than the 755 nm wave- length for blue and green ink, he adds, the PicoWay’s 532 nm laser removes RETHINKING INK see page 56 “The LFS is the only validated scale for lips that includes actual photographs of lips, rather than computer-morphed digital images, and also includes African-American females... The LFS is a clinically useful, validated and expanded scale designed for assessing lip fullness across a wide variety of races and ethnicities and goes beyond those scales previously designed for clinical trials, or computer-generated morphs. To date, the LFS is the only published scale that includes African-Americans with marked to very marked lip fullness.” Wm. Philip Werschler, M.D., Associate clinical professor of medicine and dermatology at the University of Washington SOURCE: HTTP://BIT.LY/LIPFULLNESSSCALE ES621647_DT0615_022.pgs 05.27.2015 01:25 ADVblackyellowmagentacyan
- 23. *Aminofil®,NeoGlucosamine®,andMaltobionicAcidareNeoStrata’spatentedtechnologies;Prodew®isaregisteredtrademarkofAjinomoto. ©2015NeoStrataCompany,Inc. A Breakthrough IN ANTIAGING SKIN HYDRATION NEW SKIN ACTIVE DERMAL REPLENISHMENT NEW AMINOFIL Builds skin’s natural volume to lift, firm, and reduce the appearance of lines and wrinkles NEOGLUCOSAMINE Building block of hylauronic acid plumps, diminishes spots PRODEW Provides Amino Acids essential for Natural Moisturizing Factor to hydrate MALTOBIONIC ACID Hydrates and protects against environmental, free radical damage Patented NeoStrata technologies* help reverse dehydration and visible signs of aging. ® NeoStrataPro.com|1.800.628.9904 PASSION FOR SKIN CARE. PROVEN BY SCIENCE. ® ® ES615026_DT0615_023_FP.pgs 05.16.2015 02:44 ADVblackyellowmagentacyan
- 25. OTEZLA® (apremilast) tablets, for oral use The following is a Brief Summary; refer to Full Prescribing Information for complete product information. INDICATIONS AND USAGE OTEZLA® (apremilast) is indicated for the treatment of patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy. CONTRAINDICATIONS OTEZLA is contraindicated in patients with a known hypersensitivity to apremilast or to any of the excipients in the formulation [see Adverse Reactions (6.1)]. WARNINGS AND PRECAUTIONS Depression: Treatment with OTEZLA is associated with an increase in adverse reactions of depression. Before using OTEZLA in patients with a history of depression and/or suicidal thoughts or behavior prescribers should carefully weigh the risks and benefits of treatment with OTEZLA in such patients. Patients, their caregivers, and families should be advised of the need to be alert for the emergence or worsening of depression, suicidal thoughts or other mood changes, and if such changes occur to contact their healthcare provider. Prescribers should carefully evaluate the risks and benefits of continuing treatment with OTEZLA if such events occur. During the 0 to 16 week placebo- controlled period of the 3 controlled clinical trials, 1.3% (12/920) of patients treated with OTEZLA reported depression compared to 0.4% (2/506) treated with placebo. During the clinical trials, 0.1% (1/1308) of patients treated with OTEZLA discontinued treatment due to depression compared with none in placebo-treated patients (0/506). Depression was reported as serious in 0.1% (1/1308) of patients exposed to OTEZLA, compared to none in placebo-treated patients (0/506). Instances of suicidal behavior have been observed in 0.1% (1/1308) of patients while receiving OTEZLA, compared to 0.2% (1/506) in placebo-treated patients. In the clinical trials, one patient treated with OTEZLA attempted suicide while one who received placebo committed suicide. Weight Decrease: During the controlled period of the trials in psoriasis, weight decrease between 5%-10% of body weight occurred in 12% (96/784) of patients treated with OTEZLA compared to 5% (19/382) treated with placebo. Weight decrease of ≥10% of body weight occurred in 2% (16/784) of patients treated with OTEZLA 30 mg twice daily compared to 1% (3/382) patients treated with placebo. Patients treated with OTEZLA should have their weight monitored regularly. If unexplained or clinically significant weight loss occurs, weight loss should be evaluated, and discontinuation of OTEZLA should be considered. Drug Interactions: Co-administration of strong cytochrome P450 enzyme inducer, rifampin, resulted in a reduction of systemic exposure of apremilast, which may result in a loss of efficacy of OTEZLA. Therefore, the use of cytochrome P450 enzyme inducers (e.g., rifampin, phenobarbital, carbamazepine, phenytoin) with OTEZLA is not recommended [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)]. ADVERSE REACTIONS Clinical Trials Experience in Psoriasis: Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trial of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. Diarrhea, nausea, and upper respiratory tract infection were the most commonly reported adverse reactions. The most common adverse reactions leading to discontinuation for patients taking OTEZLA were nausea (1.6%), diarrhea (1.0%), and headache (0.8%). The proportion of patients with psoriasis who discontinued treatment due to any adverse reaction was 6.1% for patients treated with OTEZLA 30 mg twice daily and 4.1% for placebo-treated patients. Table 3: Adverse Reactions Reported in ≥1% of Patients on OTEZLA and With Greater Frequency Than in Patients on Placebo; up to Day 112 (Week 16) Placebo OTEZLA 30 mg BID Preferred Term (N=506) (N=920) n (%) n (%) Diarrhea 32 (6) 160 (17) Nausea 35 (7) 155 (17) Upper respiratory tract infection 31 (6) 84 (9) Tension headache 21 (4) 75 (8) Headache 19 (4) 55 (6) Abdominal pain* 11 (2) 39 (4) Vomiting 8 (2) 35 (4) Fatigue 9 (2) 29 (3) (continued) Table 3: Adverse Reactions Reported in ≥1% of Patients on OTEZLA and With Greater Frequency Than in Patients on Placebo; up to Day 112 (Week 16) Placebo OTEZLA 30 mg BID Preferred Term (N=506) (N=920) n (%) n (%) Dyspepsia 6 (1) 29 (3) Decrease appetite 5 (1) 26 (3) Insomnia 4 (1) 21 (2) Back pain 4 (1) 20 (2) Migraine 5 (1) 19 (2) Frequent bowel movements 1 (0) 17 (2) Depression 2 (0) 12 (1) Bronchitis 2 (0) 12 (1) Tooth abscess 0 (0) 10 (1) Folliculitis 0 (0) 9 (1) Sinus headache 0 (0) 9 (1) *Two subjects treated with OTEZLA experienced serious adverse reaction of abdominal pain. Severe worsening of psoriasis (rebound) occurred in 0.3% (4/1184) patients following discontinuation of treatment with OTEZLA (apremilast). DRUG INTERACTIONS Strong CYP 450 Inducers: Apremilast exposure is decreased when OTEZLA is co-administered with strong CYP450 inducers (such as rifampin) and may result in loss of efficacy [see Warnings and Precautions (5.3) and Clinical Pharmacology (12.3)]. USE IN SPECIFIC POPULATIONS Pregnancy: Pregnancy Category C: OTEZLA should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Pregnancy Exposure Registry: There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to OTEZLA during pregnancy. Information about the registry can be obtained by calling 1-877-311-8972. Nursing Mothers: It is not known whether OTEZLA or its metabolites are present in human milk. Because many drugs are present in human milk, caution should be exercised when OTEZLA is administered to a nursing woman. Pediatric use: The safety and effectiveness of OTEZLA in pediatric patients less than 18 years of age have not been established. Geriatric use: Of the 1257 patients who enrolled in two placebo-controlled psoriasis trials (PSOR 1 and PSOR 2), a total of 108 psoriasis patients were 65 years of age and older, including 9 patients who were 75 years of age and older. No overall differences were observed in the efficacy and safety in elderly patients ≥65 years of age and younger adult patients <65 years of age in the clinical trials. Renal Impairment: OTEZLA pharmacokinetics were not characterized in patients with mild (creatinine clearance of 60-89 mL per minute estimated by the Cockcroft–Gault equation) or moderate (creatinine clearance of 30-59 mL per minute estimated by the Cockroft–Gault equation) renal impairment. The dose of OTEZLA should be reduced to 30 mg once daily in patients with severe renal impairment (creatinine clearance of less than 30 mL per minute estimated by the Cockroft–Gault equation) [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)]. Hepatic Impairment: Apremilast pharmacokinetics were characterized in patients with moderate (Child Pugh B) and severe (Child Pugh C) hepatic impairment. No dose adjustment is necessary in these patients. OVERDOSAGE In case of overdose, patients should seek immediate medical help. Patients should be managed by symptomatic and supportive care should there be an overdose. Manufactured for: Celgene Corporation, Summit, NJ 07901 OTEZLA® is a registered trademark of Celgene Corporation. Pat. http://www.celgene.com/therapies ©2014 Celgene Corporation, All Rights Reserved. Based on APRPI.003 OTZ_PsO_HCP_BSv.003 09_2014 Rx Only ES621893_DT0615_025_FP.pgs 05.27.2015 04:03 ADVblack
- 26. ® JUNE 2015 ⁄ DERMATOLOGYTIMES.COM 26 COSMETIC DERMATOLOGY surgical neck contouring and lifting procedure.Theresultsarepermanent, asthedrugiscytotoxictothefatcells,” Dr.ToroktellsDermatologyTimes.The drugisnotapprovedorrecommended for use outside the submental area. “When properly injected into sub- mental fat, the drug destroys fat cells; however,itcanalsodestroyothertypes of cells, such as skin cells, if it is inad- vertently injected into the skin,” ac- cording to the FDA’s release. Dermatologists can use up to 50 injections in a treatment and up to six single treatments given a month apart. Kybella’s single-patient-use vials should not be diluted or mixed with other compounds. Common side effects associated with Kybella include swelling, bruis- ing, pain, numbness, redness and hard areas where patients have been treated. Dermatologists should note that Kybella can cause serious side ef- fects. One such side effect is nerve in- juryinthejaw,whichcanresultintrou- ble swallowing, facial weakness or an uneven smile. “The expected side effects of swell- ing, redness and pain are the result of the drug having its expected effect on the cells and this resolves over a pe- riod of several weeks,” Dr. Torok says. Dermatologists should use caution in patients who have had prior surgical or aesthetic treatment of the submen- tal area. And if there is no distinctive hologramontheviallabel,dermatolo- gists are urged not to use the product. “Treatment with Kybella should only be provided by a licensed health care professional, and patients should fully understand the risks associated with use of the drug before consid- ering treatment,” says Amy G. Egan, M.D., M.P.H., a deputy director in the FDA’s Center for Drug Evaluation and Research. DT SHRINK DOUBLE CHINS WITHOUT SURGERY Injectable approved for submental fat from page 1 N –AsAmericans’taste fortattooshasgrown,expertssay,sohave many tattoo wearers’ regrets. Nationally, says Eric F. Bernstein, M.D.,M.S.E.,“There’satotalepidemicof people wanting their tattoos removed.” He is clinical professor, Department of Dermatology, University of Pennsylva- nia School of Medicine. George Hruza, M.D., M.B.A., says he commonly sees parents who want tat- toos removed from children — who got themwithoutpermission—asyoungas 16. He is a Chesterfield, Missouri-based dermatologist in private practice. Dr. Bernsteinaddsthathe’sremovedrecent tattoosfrompatientsfrom14yearsoldto senior citizens. Regardingtattoolocations,Dr.Hruza says that as tattoos have grown more mainstream,heincreasinglyzapsthem from highly visible areas such as the neck,oncethewearersrethinktheirink. Roy Geronemus, M.D., adds that as people become more aware that lasers can safely and effectively remove tat- toos, he sees growing numbers of pa- tients with lip and eyeliner tattoos they wantremoved.HeisdirectoroftheLaser & Skin Surgery Center of New York and clinicalprofessorofdermatologyatNew York University Medical Center. Treating tattooed-on cosmetics can betricky,however.Frequently,explains Dr. Hruza, they contain iron oxide (for tan and rust tones) or titanium diox- ide (for pastels and flesh tones). Imme- diately after Q-switched or picosecond laser treatment, he says, both materials oftenirreversiblydarkentograyorpitch- blacktonesasrefractoryasgenuineblack ink.Dr.Bernsteinsaysthedarkenedpig- mentcantakedoubleortripletheusual Tattoo removal Tattoo removal is booming, experts say, even among patients at extremes of the age spectrum. However, removing trendy lip- and eyeliner tattoos can be tricky because they often contain iron oxide or titanium dioxide. QUICK READJOHN JESITUS | STAFF CORRESPONDENT “Thetattoo-removal businessisbooming —andit’sonlygoing tokeepboomingas timegoeson.” Eric F. Bernstein, M.D. Ardmore, Penn. numberofQ-switchedlasertreatments. Whenusingsuchlasersforfacialcos- metic tattoos, particularly with rusty hues,Dr.Hruzarecommendsfirsttreat- ing a small test spot — which one can surgically excise if it darkens. Alterna- tively, Dr. Geronemus uses an ablative CO2 or fractional ablative CO2 laser for tattoos containing iron oxide or tita- nium dioxide. Dr. Bernstein says that although he has not yet treated cosmetic tattoos with the PicoWay 532/1064 nm pico- second laser (Syneron & Candela), he hopes that it simplifies the task. For now, he says that as tattooed Ameri- cansgetolderandperhapswiser,“The tattoo-removalbusinessisbooming— and it’s only going to keep booming as time goes on.” DT Disclosures: Dr. Geronemus is a clinical investigator for Cynosure, Syneron & Candela and Cutera. Dr. Ber- nstein is head of Syneron & Candela’s medical advi- sory board and received a research grant from the company to perform the PicoWay Phase III trial. Dr. Hruza reports no relevant financial interests. A booming business “There’s a total epidemic of people wanting their tattoos removed.” Eric F. Bernstein, M.D. Ardmore, Penn. ES618076_DT0615_026.pgs 05.22.2015 21:14 ADVblackyellowmagentacyan
- 27. Introducing RETIN-A MICRO ® (tretinoin) Gel microsphere, Exclusively available in a 50g pump Except as otherwise indicated, all product names, slogans, and other marks are trademarks of the Valeant family of companies. © 2014 Valeant Pharmaceuticals North America LLC. DM/RAM/14/0003 06/14 Printed in USA. NEW STRENGTH!
- 28. Introducing RETIN-A MICRO ® (tretinoin) Gel microsphere, Exclusively available in a 50g pump Except as otherwise indicated, all product names, slogans, and other marks are trademarks of the Valeant family of companies. © 2014 Valeant Pharmaceuticals North America LLC. DM/RAM/14/0003 06/14 Printed in USA. NEW STRENGTH!
- 29. 29JUNE 2015 ⁄ DERMATOLOGYTIMES.COM COSMETIC DERMATOLOGY A approved for use in asthma inhalers is reported to begin phase 3 trials during the first half of 2015 for use as an injectable option in fat reduction and body contouring. LIPO-202 (Neothetics) is a physician- administered injectable form of sal- meterolxinafoate,abeta-2adrenergic receptor that not only helps asthmat- ics breathe better but also shrinks fat cells, according to researchers. Marina Peredo, M.D., a derma- tologist in Manhattan and Long Island, N.Y., and clinical investigator for LIPO 202, says physicians can per- form the nonsurgical procedure in less than 5 minutes. The procedure includes making 20 injections, about 4 cm apart, at the site of an abdom- inal fat bulge. Patients come back weekly, for a total of 8 weeks. Often by the first month, she says, they notice a circumference change in their bodies. It’s painless and requires no downtime. “I think this product is going to do to body contouring or body sculpt- ing what Botox did to facelifts,” Dr. Peredo says. LIPO-202 PHASE 2 RESULTS Based on the clinical phase 2 stud- ies, LIPO-202 is effective at reduc- ing abdominal fat and has a side ef- fect profile virtually identical to pla- cebo, says Mark Nestor, M.D., Ph.D., a clinical investigator in LIPO-202 phase 2 clinical trials and co-chair of Neothetics’ scientific advisory board. “Patients in the study appear to be very satisfied with the results,” says Dr. Nestor, director for the Cen- ter for Clinical and Cosmetic Re- search and the Center for Cosmetic Enhancement in Aventura, Fla., and voluntar y associate professor, dermatology and cutaneous surgery, University of Miami Miller School of Medicine, Miami, Fla. Neothetics, formerly Lithera, an- nounced results from its phase 2b RESET clinical study of LIPO-202 in a September 30, 2013, press release. Researchers tested three doses (0.4, 1.0 and 4.0 µg) in a multicenter, ran- domized, placebo-controlled trial, including 513 healthy, nonobese people with abdominal bulging due to excess subcutaneous fat. Ideal candidates were those bothered by slight to moderate abdominal bulges, according to Dr. Peredo. “If they were too heavy, this was not an indication, at least for this particular study,” she says. An additional exclusion criterion was use of inhalers because it’s the same medication, according to Dr. Peredo. Researchers found the optimal treatment dose was 0.4 µg (20 times 0.02 µg/mL). Adverse events with LIPO-202 were generally mild, tran- sientandsimilartoplacebo.Themost common adverse event was injection site reactions, according to the press release, and no patients withdrew from the study for this reason. Asforefficacy,“Responderanalyses usingacompositeendpointofapatient self-assessment(5-pointverbalPatient Global Abdominal Profile Scale – P- GAPS)plusaclinicianratingofabdom- inal contour (6-point visual Clinician Photo-numeric Scale – CPnS) demon- stratedsignificantefficacyofLIPO-202 at the 0.4 µg dose versus placebo…,” according to the release. Patients in the treatment group showed a mean reduction in cir- cumference at the umbilicus of 1.6 cm versus 0.65 cm for placebo. The average reduction in abdominal vol- ume in the treatment zone was 192 cc for the 0.4 µg LIPO-202 dose versus 68 cc for placebo. SALMETEROL SAFETY PROFILE The inhaled form of salmeterol, one of the medicines found in Advair (GSK), hasalistofsideeffectsrangingfromthe more common — shortness of breath and chest tightness — to the more dangerous, including irregular heart- beat.Unexplainedweightlossisoneof the less common side effects. Dr.Nestorsayssalmeterolxinafoate is a widely used, very safe medication forlungdiseaseandhasbeenapproved for that indication for many years. “Studies have show n a side effect profile similar to placebo when inhaled directly resulting in much higherbloodlevelsthancanbeachieved by the amount utilized in clinical LIPO-202 see page 34 LIPO-202 enters Phase 3 trials for injectable fat reduction LISETTE HILTON | STAFF CORRESPONDENT LIPO-202is effectiveatreducing abdominalfatand hasasideeffect profilevirtually identicaltoplacebo. It’spainless andrequiresno downtime. “I think this product is going to do to body contouring or body sculpting what Botox did to facelifts. Injecting this substance bolsters metabolism. We’re not killing fat cells; we’re actually just shrinking them.” Marina Peredo, M.D. Long Island, N.Y. ES617947_DT0615_029.pgs 05.22.2015 02:34 ADVblackyellowmagentacyan