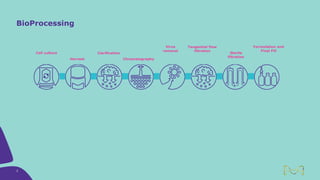
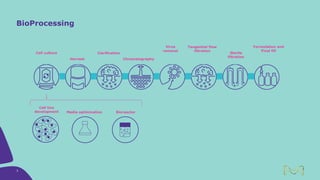
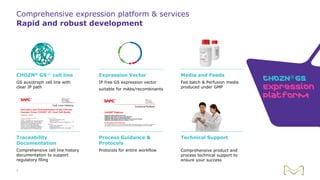
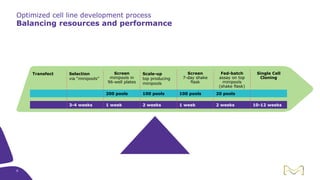
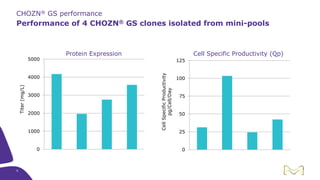
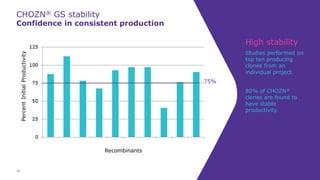
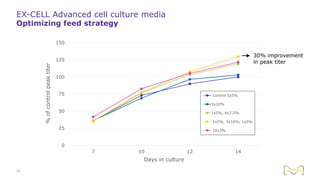
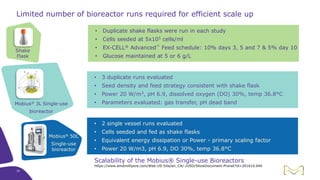
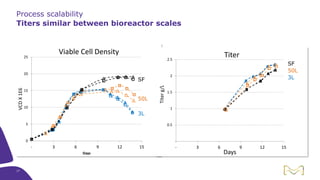
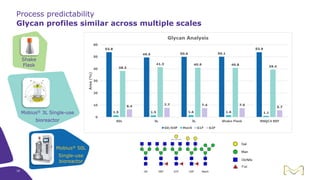
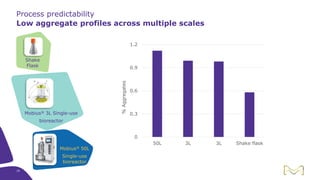
Joe Orlando presented on Merck KGaA's platforms for robust and scalable upstream bioprocess development. Their CHOZN GS expression system provides a stable recombinant cell line, optimized media and feeds, and single-use bioreactors to efficiently develop and scale processes from shake flasks to 50L bioreactors. Using their TNFR Fc-fusion protein as an example, Merck demonstrated consistent titers, glycan profiles, and low aggregates across scales. Their turnkey solution aims to reduce development time and improve product quality and consistency throughout the upstream process.