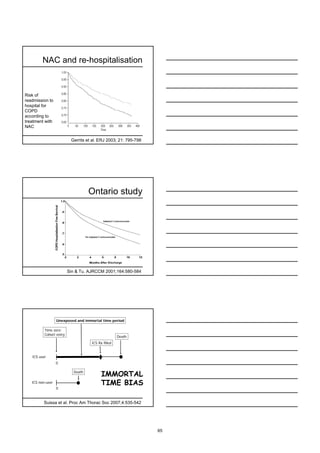
The document discusses the role of oral mucolytics in the prevention of lower respiratory tract infections (LRTIs) in patients with chronic obstructive pulmonary disease (COPD). It summarizes the findings of clinical trials that show mucolytics reduce exacerbations in COPD patients not treated with inhaled corticosteroids. However, the largest trial (BRONCUS) found no difference in exacerbation rates between N-acetylcysteine and placebo in COPD patients, though a subgroup analysis found fewer exacerbations in patients not on inhaled corticosteroids with N-acetylcysteine. Given most COPD patients are now treated with inhaled corticosteroids, the role of mucolytics in