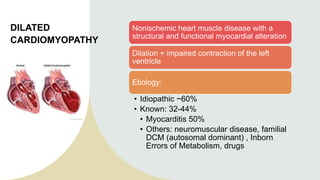
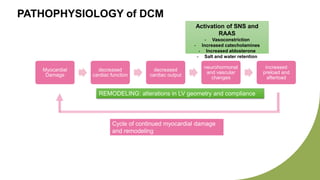
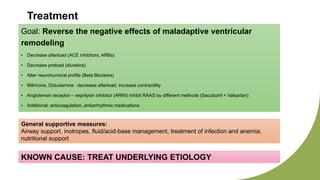
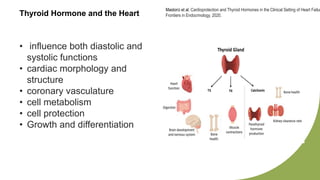
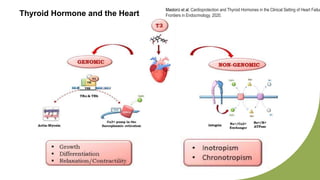
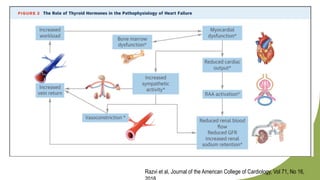
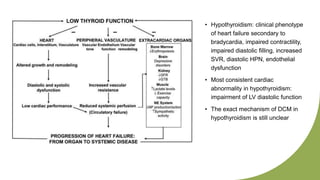
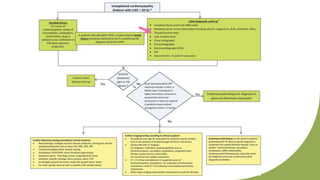
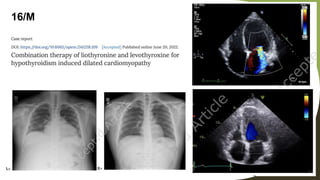
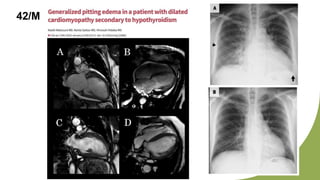
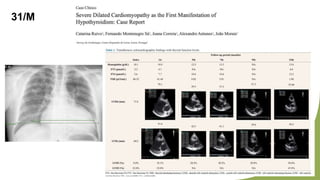
Dilated cardiomyopathy can be caused by hypothyroidism. Hypothyroidism leads to structural and functional changes in the heart muscle including dilation and impaired contraction of the left ventricle. While most cases of dilated cardiomyopathy have unknown causes, treatment of underlying causes like hypothyroidism can help reverse ventricular remodeling and improve cardiac function. Thyroid hormones influence cardiac morphology, function, metabolism and growth. Assessing thyroid function is therefore recommended for all patients presenting with heart failure to identify potentially treatable secondary causes like hypothyroidism.