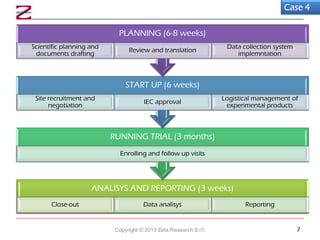
An Italian medical device manufacturer wanted to conduct a post-market clinical trial abroad to obtain marketing data quickly. A contract research organization proposed conducting the randomized controlled trial at an investigational site in an extra-EU country to maximize resources and expedite the 6-7 month timeline for analyzed data. The CRO planned to translate and adapt the study documents to local requirements, monitor the site, obtain ethics approval, manage products on site, and analyze data remotely according to good clinical practice to meet the client's goals in a cost-effective manner.