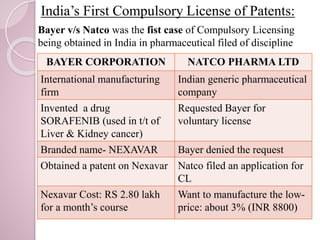
This document discusses compulsory licensing of patents in India. Compulsory licensing allows the government to grant a license to a third party to produce a patented product or use a patented process without the consent of the patent holder. The objectives are to reward patent holders while also making patented pharmaceutical products available to the public at affordable prices. Section 84 of the Indian Patent Act lays out the conditions for granting compulsory licenses, such as if the patented invention is not available to the public at a reasonable price. The first compulsory license granted in India was for the cancer drug Nexavar, allowing generic manufacturer Natco to sell a version at a much lower price than the branded version from Bayer.