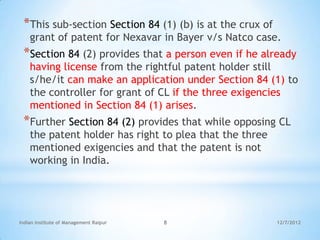
The document summarizes the first case of compulsory licensing granted in India, between Bayer and Natco Pharmaceuticals regarding the drug Nexavar. The Controller of Patents granted the license to Natco after determining that Bayer's drug was not reasonably affordable in India. Some key points:
- Bayer's Nexavar treatment cost over $2,000/month while Natco's generic version cost $88/month.
- Bayer had not manufactured the drug in India or made it widely available.
- The license allows Natco to produce a generic version at a significantly lower cost, while still paying a 6% royalty to Bayer.




![*Following is the brief mention of the provisions of
section 84 and 92 of the Act.
*Section 84 of Compulsory licenses:
*At any time after the expiration of three years from
the date of the [grant] of a patent, any person
interested may make an application to the Controller
for grant of compulsory license on patent on any of
the following grounds, namely:—
*(a) That the reasonable requirements of the public
with respect to the patented invention have not been
satisfied, or
*(b) That the patented invention is not available to
the public at a reasonably affordable price, or
*(c) That the patented invention is not worked in the
territory of India.
Indian Institute of Management Raipur 6 12/7/2012](https://image.slidesharecdn.com/bayervsnatcofinal-121207130144-phpapp02/85/Bayer-vs-Natco-Case-6-320.jpg)