Clinical Compliance Solutions Summary
•Download as DOCX, PDF•
1 like•479 views
Clinical Compliance Solutions is a company founded by Sharon Reinhard to provide regulatory and compliance solutions to small and mid-sized biotech and pharmaceutical companies. Ms. Reinhard has 18 years of experience in clinical development, operations, compliance and quality assurance at large companies. She offers interim leadership, gap analyses, SOP development, inspection readiness strategies, audits and training to help clients stay compliant with evolving standards. Her services are customized based on a company's size, maturity and complexity.
Report
Share
Report
Share
Recommended
Solutions for your pharmaco genetic testing & web site link

Now that knowing your DNA/PharmacoGenetics is key to a lifetime benefit, see the Best Practices Solution, while making some additional dollars in reimbursement revenues.
Adaptive Designs for Medical Device Clinical Studies - By Compliance Global ...

Adaptive Designs for Medical Device Clinical Studies - By Compliance Global ...Compliance Global Inc
US and EU regulatory requirements for adaptive clinical trials, its rationale, logistics and differences for drug, biologic and medical devicesVendor Selection & Management - By AtoZ Compliance

Selection process for potential vendors, strategies for management and risk mitigation approach for key vendors
Multiple Contract Pharmacies in a Disproportionate Share Hospital System

APhA2010 Presentation on Multiple Contract Pharmacies
Recommended
Solutions for your pharmaco genetic testing & web site link

Now that knowing your DNA/PharmacoGenetics is key to a lifetime benefit, see the Best Practices Solution, while making some additional dollars in reimbursement revenues.
Adaptive Designs for Medical Device Clinical Studies - By Compliance Global ...

Adaptive Designs for Medical Device Clinical Studies - By Compliance Global ...Compliance Global Inc
US and EU regulatory requirements for adaptive clinical trials, its rationale, logistics and differences for drug, biologic and medical devicesVendor Selection & Management - By AtoZ Compliance

Selection process for potential vendors, strategies for management and risk mitigation approach for key vendors
Multiple Contract Pharmacies in a Disproportionate Share Hospital System

APhA2010 Presentation on Multiple Contract Pharmacies
Abpi launched clinical-trial_disclosure_toolkit

Keeping with its promises, Association of the British Pharmaceutical Industry has launched a clinical-trial disclosure toolkit to help its member businesses observe with transparency needs for information from or about clinical trials.
Abpi launched clinical trial disclosure toolkit

Keeping with its promises, Association of the British Pharmaceutical Industry has launched a clinical-trial disclosure toolkit to help its member businesses observe with transparency needs for information from or about clinical trials.
How To Boost Hospital Performance By Optimizing Your Pharmacy

Assessing and managing productivity is a complex process that takes the rights tools and people. While pharmacy may seem to be a small part of an overall organization, it is actually one of the largest cost centers of a hospital, making it one of the most important departments to optimize and streamline. Learn how your pharmacy’s productivity can impact your hospital’s overall costs, quality, safety and patient satisfaction.
Key Points:
- Analyzing productivity
- Pros and cons of pharmacy productivity management tools
- Use of volume indicators
- Workflows to improve productivity and communication with nursing and hospital staff
Five Questions to Ask a Prospective CDM Vendor

CURO Clinical Data Management is a full-service clinical data management contract research organization (CRO) supporting Phase I-IV clinical trials for the global biopharmaceutical industry.
The data generated during Phase I-IV clinical studies is essential to the ultimate success of an investigational product. The data management vendor you choose must recognize and respect the importance of this central function.
Surveyors are Here

Do you feel like there are more regulatory and accrediting surveys being conducted than ever before? Have you been the host of a CMS survey…or two… in the last several years? Do you feel like the surveys are becoming more onerous or prescriptive?
Dismantling Barriers to Adaptive Design Adoption to Be Key Theme of Pre-Disru...

Dismantling Barriers to Adaptive Design Adoption to Be Key Theme of Pre-Disruptive Innovations Conference
Assuring quality improvement - Jerome Ng - Waitemata DHB

Award finalist for Ko Awatea International Excellence in Health Improvement Awards - Jerome Ng, Waitemata District Health Board
ADHB's Solution for Radiology Order Entry and Results Signoff

Dr Charles Bradfield
Auckland District Health Board
www.adhb.govt.nz
(P35, 1/10/09, Works Room, 11.13)
Current regulatory expectation

The webinar discusses current regulatory expectation on how a firm identifies deviations, investigate the cause, recommend corrective, preventive actions.
Getting Started with Bundled Payments for Orthopedics

The webinar will provide a basic outline of a bundled payment model for total joint replacement or other orthopedic procedures, based on the experience at Hoag with real life examples of problems encountered and solutions created. The session will provide an overview of what to consider for process and contracting, including who should be involved in the development process and how to mitigate risk in the model.
About the Speaker:
Gabrielle White, RN, CASC, has over 23 years experience in the health care industry with a background in peri operative nursing and ASC administration. She is currently an Executive Director at Hoag Orthopedic Institute where she has clinical, business and administrative oversight of the Orthopedic Surgery Center of Orange County and leads Network Development for Hoag Orthopedic Institute with a primary focus on bundled payment programs and growth strategy with self-insured employers.
What is Medical Auditing? How it can be Performed?

Medical auditing entails conducting internal or external reviews of coding accuracy, policies, and procedures to ensure you are running an efficient and hopefully liability-free operation. Quality health care is based on accurate and complete clinical documentation in the medical record. The best way to improve your clinical documentation and the livelihood of your health care organization is through medical record audits.
Strategies for Conducting New Product Scientific Assessment - Yavuz SILAY - D...

Strategies for Conducting New Product Scientific Assessment - Due Diligence - New Strategies for Successful Licensing Acquisitions , DIA , Session Panel, June 22 2008,
More Related Content
What's hot
Abpi launched clinical-trial_disclosure_toolkit

Keeping with its promises, Association of the British Pharmaceutical Industry has launched a clinical-trial disclosure toolkit to help its member businesses observe with transparency needs for information from or about clinical trials.
Abpi launched clinical trial disclosure toolkit

Keeping with its promises, Association of the British Pharmaceutical Industry has launched a clinical-trial disclosure toolkit to help its member businesses observe with transparency needs for information from or about clinical trials.
How To Boost Hospital Performance By Optimizing Your Pharmacy

Assessing and managing productivity is a complex process that takes the rights tools and people. While pharmacy may seem to be a small part of an overall organization, it is actually one of the largest cost centers of a hospital, making it one of the most important departments to optimize and streamline. Learn how your pharmacy’s productivity can impact your hospital’s overall costs, quality, safety and patient satisfaction.
Key Points:
- Analyzing productivity
- Pros and cons of pharmacy productivity management tools
- Use of volume indicators
- Workflows to improve productivity and communication with nursing and hospital staff
Five Questions to Ask a Prospective CDM Vendor

CURO Clinical Data Management is a full-service clinical data management contract research organization (CRO) supporting Phase I-IV clinical trials for the global biopharmaceutical industry.
The data generated during Phase I-IV clinical studies is essential to the ultimate success of an investigational product. The data management vendor you choose must recognize and respect the importance of this central function.
Surveyors are Here

Do you feel like there are more regulatory and accrediting surveys being conducted than ever before? Have you been the host of a CMS survey…or two… in the last several years? Do you feel like the surveys are becoming more onerous or prescriptive?
Dismantling Barriers to Adaptive Design Adoption to Be Key Theme of Pre-Disru...

Dismantling Barriers to Adaptive Design Adoption to Be Key Theme of Pre-Disruptive Innovations Conference
Assuring quality improvement - Jerome Ng - Waitemata DHB

Award finalist for Ko Awatea International Excellence in Health Improvement Awards - Jerome Ng, Waitemata District Health Board
ADHB's Solution for Radiology Order Entry and Results Signoff

Dr Charles Bradfield
Auckland District Health Board
www.adhb.govt.nz
(P35, 1/10/09, Works Room, 11.13)
Current regulatory expectation

The webinar discusses current regulatory expectation on how a firm identifies deviations, investigate the cause, recommend corrective, preventive actions.
Getting Started with Bundled Payments for Orthopedics

The webinar will provide a basic outline of a bundled payment model for total joint replacement or other orthopedic procedures, based on the experience at Hoag with real life examples of problems encountered and solutions created. The session will provide an overview of what to consider for process and contracting, including who should be involved in the development process and how to mitigate risk in the model.
About the Speaker:
Gabrielle White, RN, CASC, has over 23 years experience in the health care industry with a background in peri operative nursing and ASC administration. She is currently an Executive Director at Hoag Orthopedic Institute where she has clinical, business and administrative oversight of the Orthopedic Surgery Center of Orange County and leads Network Development for Hoag Orthopedic Institute with a primary focus on bundled payment programs and growth strategy with self-insured employers.
What is Medical Auditing? How it can be Performed?

Medical auditing entails conducting internal or external reviews of coding accuracy, policies, and procedures to ensure you are running an efficient and hopefully liability-free operation. Quality health care is based on accurate and complete clinical documentation in the medical record. The best way to improve your clinical documentation and the livelihood of your health care organization is through medical record audits.
What's hot (19)
How To Boost Hospital Performance By Optimizing Your Pharmacy

How To Boost Hospital Performance By Optimizing Your Pharmacy
Dismantling Barriers to Adaptive Design Adoption to Be Key Theme of Pre-Disru...

Dismantling Barriers to Adaptive Design Adoption to Be Key Theme of Pre-Disru...
Assuring quality improvement - Jerome Ng - Waitemata DHB

Assuring quality improvement - Jerome Ng - Waitemata DHB
ADHB's Solution for Radiology Order Entry and Results Signoff

ADHB's Solution for Radiology Order Entry and Results Signoff
Getting Started with Bundled Payments for Orthopedics

Getting Started with Bundled Payments for Orthopedics
What is Medical Auditing? How it can be Performed?

What is Medical Auditing? How it can be Performed?
Similar to Clinical Compliance Solutions Summary
Strategies for Conducting New Product Scientific Assessment - Yavuz SILAY - D...

Strategies for Conducting New Product Scientific Assessment - Due Diligence - New Strategies for Successful Licensing Acquisitions , DIA , Session Panel, June 22 2008,
Effective Pharmaceutical Life Cycle Management Planning: Optimizing Product V...

Developing an effective drug life cycle management plan is critical for maximizing the value of a pharma product over the course of its commercial lifespan. Drawing from our deep expertise of pharmaceutical product lifecycle management, RxC International, a life sciences management consulting firm, has identified key success factors and industry best practices as well as common pitfalls to avoid when developing and implementing LCM plans.
Clinical Research Organization Services | Contract Research Company - Pepgra

Pepgra is a global contract research organization and drug development services company. It provides various phases of clinical research trials services to pharmaceutical and biotechnology companies to help reduce the time and costs associated with drug development.
Contact Us:
Website : https://bit.ly/33Fwsye
Email us: sales.cro@pepgra.com
India: +91 9884350006
United Kingdom: +44- 74248 10299
Shamisha corporate presentation 10112016

Shamisha Learning Center, Ahmedabad, Gujarat, India-Specialized Training, Workshops and courses on Quality, Regulatory, R & D, Supply Chain and Manufacturing
Sai pharma consultants

T.Rama Rao is a Sr.Consultant & Promoter of M/s.Saipharma Consultancy with Team Members having rich experience in handlig Project Management, Audits & Compliance Management, Regulatory Affairs, Pharmaceutical Product Development, GxP Training/ Operational Excellence
We work with Micro ,Small ,Medium and Large Enterprises in Pharma Formulations/Intermediates ,APIs,& Chemicals / Medical Devices/ BioTech / AYUSH/ Primary Pkg Mtrs/ Clinical Research Organisation .
Please visit our web site www.saipharmaconsultants.com for further details
4.Out sourcing BA&BE to CRO

Outsourcing the bioavailability and bioequivalnce to Contract research organisation
Advance Your Program in China with Fully Integrated Clinical Development Solu...

Meet your needs for greater efficiency, expanded capacity and customized support at the newly opened Covance Drug Development Center in Shanghai, China.
ACMQ NoonConference100527

Slide Deck for Chapter 6 in Medical Quality Management, published by the American College of Medical Quality.
Product Complaints: Complaint Handling from Intake to Closure

Overview :
An effective complaint handling system is an extremely important part of any quality system whether you are a medical device or pharmaceutical manufacturer. Manufacturers should understand that any complaint received on a product shall be evaluated and, if necessary, thoroughly investigated and analyzed, and corrective action shall be taken. Manufacturers must ensure that they have a well-designed system to address complaints related to their products. Components of a well-designed system include: process/procedure, trained personnel, and proper record keeping. Complaint handling is no easy task. Management might overlook the importance of customer feedback and be unable to capture all complaints coming from disparate sources. Plus, the additional regulatory reporting requirement related to adverse events may seem overly burdensome to device makers in particular. A strategic risk-based methodology can help streamline the complaint-handling process in medical device manufacturing.
Similar to Clinical Compliance Solutions Summary (20)
Strategies for Conducting New Product Scientific Assessment - Yavuz SILAY - D...

Strategies for Conducting New Product Scientific Assessment - Yavuz SILAY - D...
Effective Pharmaceutical Life Cycle Management Planning: Optimizing Product V...

Effective Pharmaceutical Life Cycle Management Planning: Optimizing Product V...
Clinical Research Organization Services | Contract Research Company - Pepgra

Clinical Research Organization Services | Contract Research Company - Pepgra
Advance Your Program in China with Fully Integrated Clinical Development Solu...

Advance Your Program in China with Fully Integrated Clinical Development Solu...
Highlights from ExL Pharma's 4th Latin America Clinical Trials

Highlights from ExL Pharma's 4th Latin America Clinical Trials
Product Complaints: Complaint Handling from Intake to Closure

Product Complaints: Complaint Handling from Intake to Closure
Clinical Compliance Solutions Summary
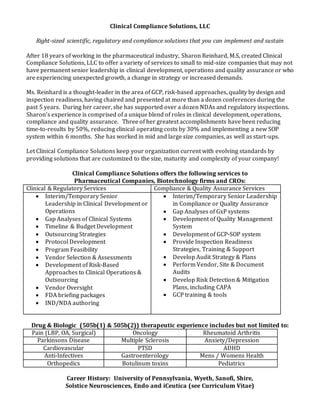
- 1. Clinical Compliance Solutions, LLC Right-sized scientific, regulatory and compliance solutions that you can implement and sustain After 18 years of working in the pharmaceutical industry, Sharon Reinhard, M.S, created Clinical Compliance Solutions, LLC to offer a variety of services to small to mid-size companies that may not have permanent senior leadership in clinical development, operations and quality assurance or who are experiencing unexpected growth, a change in strategy or increased demands. Ms. Reinhard is a thought-leader in the area of GCP, risk-based approaches, quality by design and inspection readiness, having chaired and presented at more than a dozen conferences during the past 5 years. During her career, she has supported over a dozen NDAs and regulatory inspections. Sharon’s experience is comprised of a unique blend of roles in clinical development, operations, compliance and quality assurance. Three of her greatest accomplishments have been reducing time-to-results by 50%, reducing clinical operating costs by 30% and implementing a new SOP system within 6 months. She has worked in mid and large size companies, as well as start-ups. Let Clinical Compliance Solutions keep your organization current with evolving standards by providing solutions that are customized to the size, maturity and complexity of your company! Clinical Compliance Solutions offers the following services to Pharmaceutical Companies, Biotechnology firms and CROs: Clinical & Regulatory Services Compliance & Quality Assurance Services Interim/Temporary Senior Leadership in Clinical Development or Operations Gap Analyses of Clinical Systems Timeline & Budget Development Outsourcing Strategies Protocol Development Program Feasibility Vendor Selection & Assessments Development of Risk-Based Approaches to Clinical Operations & Outsourcing Vendor Oversight FDA briefing packages IND/NDA authoring Interim/Temporary Senior Leadership in Compliance or Quality Assurance Gap Analyses of GxP systems Development of Quality Management System Development of GCP-SOP system Provide Inspection Readiness Strategies, Training & Support Develop Audit Strategy & Plans Perform Vendor, Site & Document Audits Develop Risk Detection & Mitigation Plans, including CAPA GCP training & tools Drug & Biologic (505b(1) & 505b(2)) therapeutic experience includes but not limited to: Pain (LBP, OA, Surgical) Oncology Rheumatoid Arthritis Parkinsons Disease Multiple Sclerosis Anxiety/Depression Cardiovascular PTSD ADHD Anti-Infectives Gastroenterology Mens / Womens Health Orthopedics Botulinum toxins Pediatrics Career History: University of Pennsylvania, Wyeth, Sanofi, Shire, Solstice Neurosciences, Endo and iCeutica (see Curriculum Vitae)