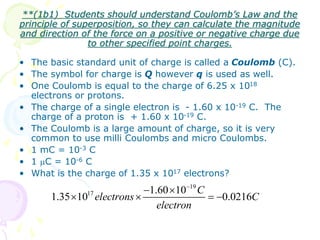
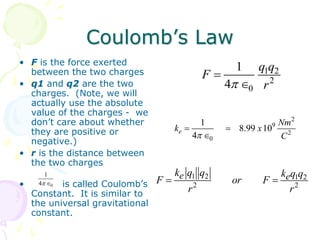
1. Coulomb's law describes the electrostatic force of attraction or repulsion between two point charges. The magnitude of the force is directly proportional to the product of the charges and inversely proportional to the square of the distance between them.
2. The superposition principle states that when multiple charges exert forces on a single charge, the net electrostatic force is calculated by vector addition of the individual forces.
3. In problems involving multiple charges, the individual forces must be resolved into x and y components and then combined vectorially to find the net force and direction.