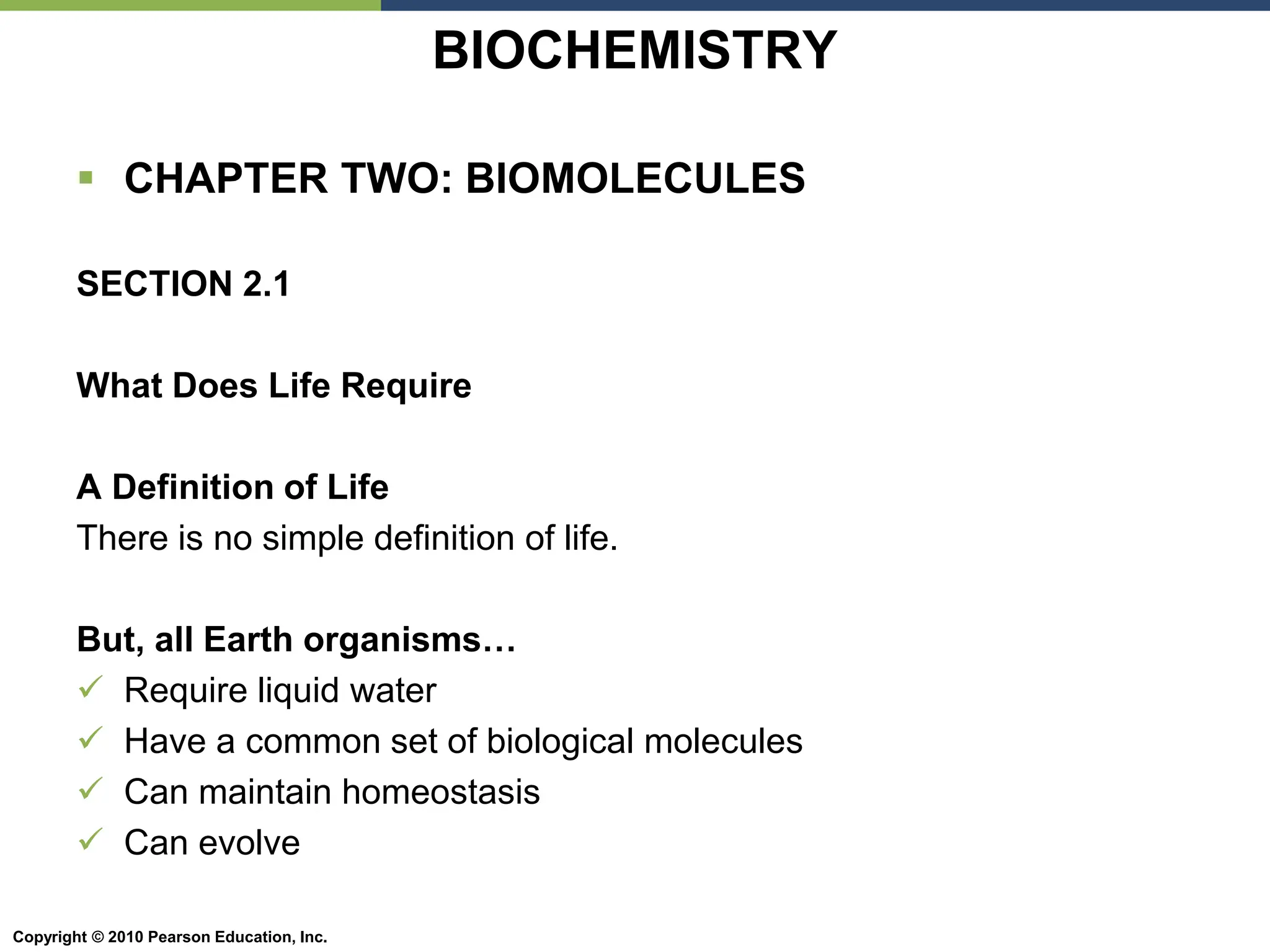
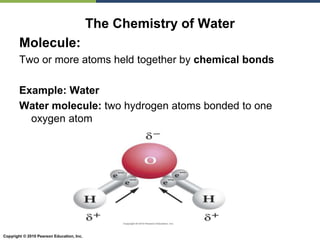
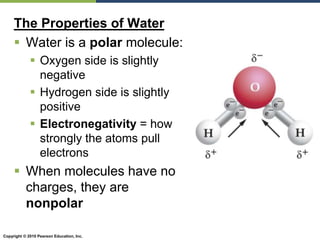
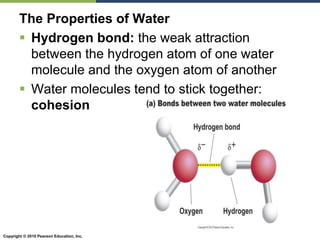
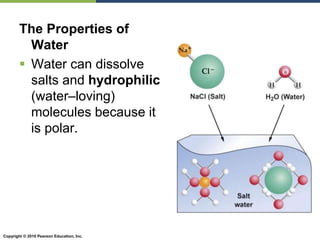
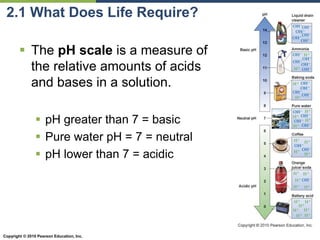
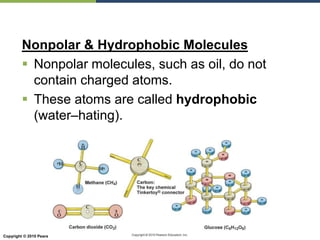
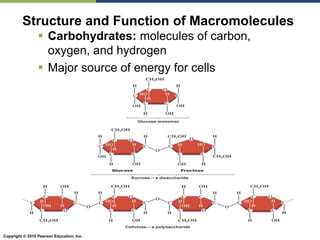
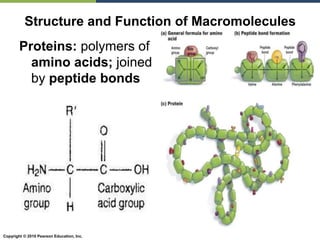
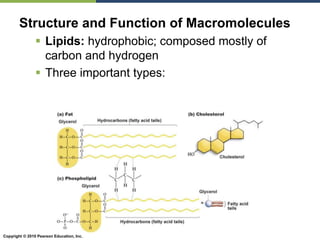
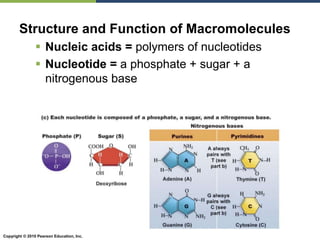
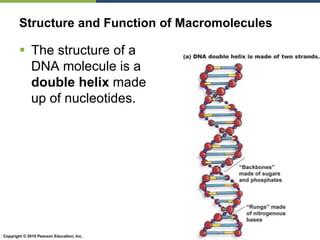
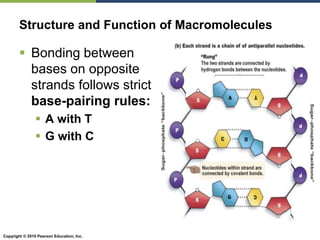
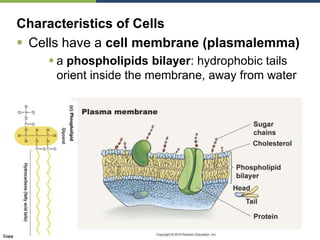
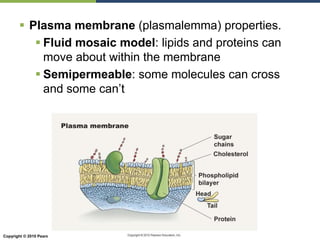
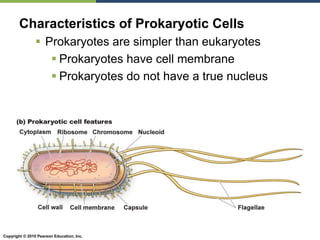
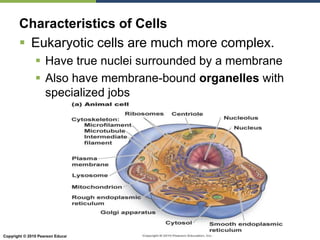
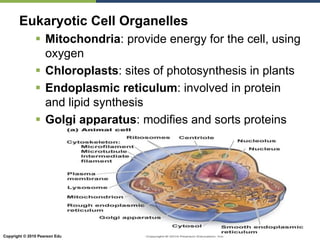
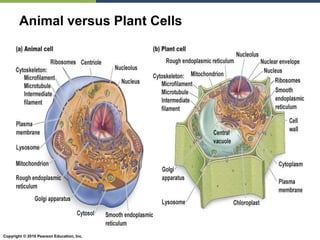
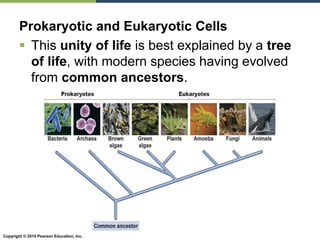
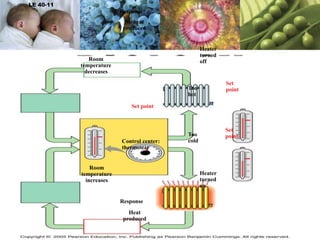
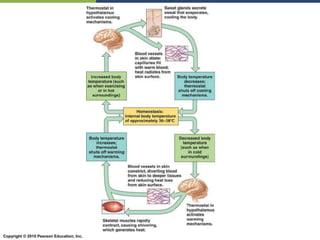
The document discusses the key requirements for life, including water, common biological molecules, homeostasis, and evolution. It explains that all living things are made of cells that use organic macromolecules like carbohydrates, proteins, lipids, and nucleic acids. The document contrasts prokaryotic and eukaryotic cells and describes their organelles. It introduces homeostasis as the process by which organisms maintain stable internal conditions and regulate physiological parameters through feedback systems.