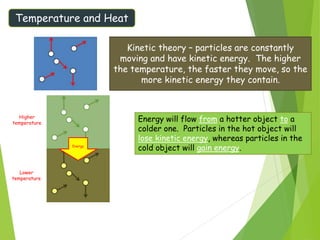
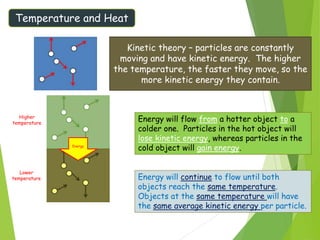
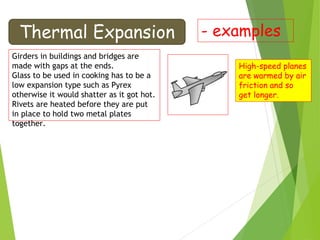
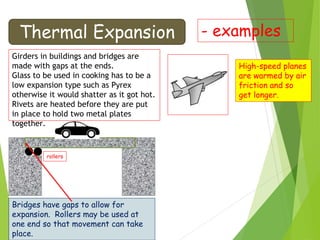
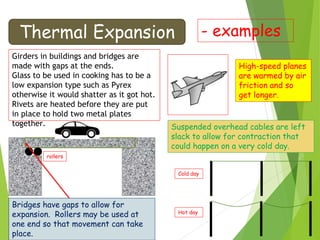
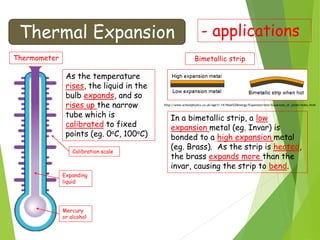
This document discusses various topics related to thermal properties of matter, including:
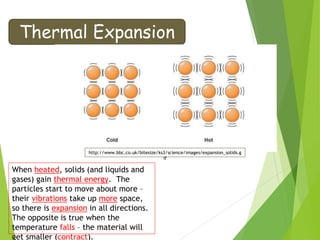
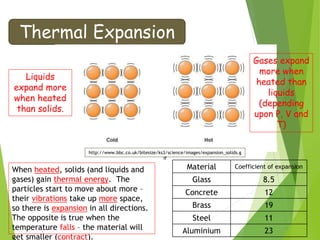
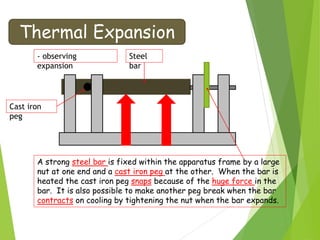
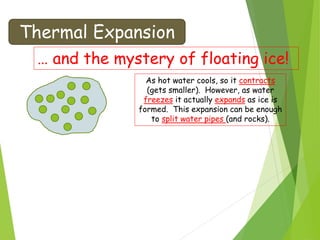
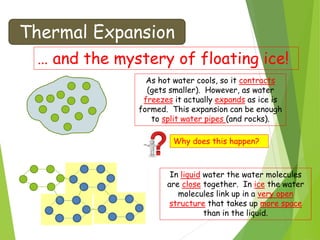
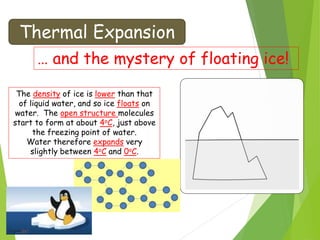
- Thermal expansion of solids, liquids and gases and its everyday applications.
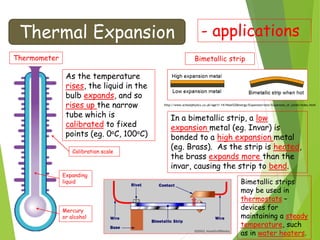
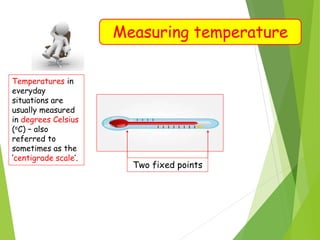
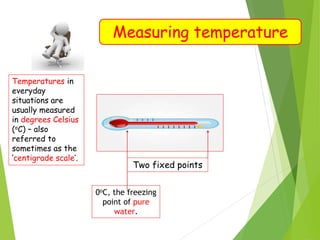
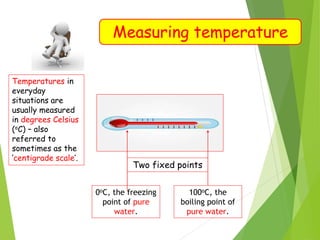
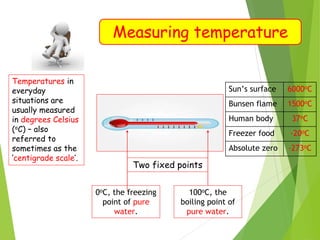
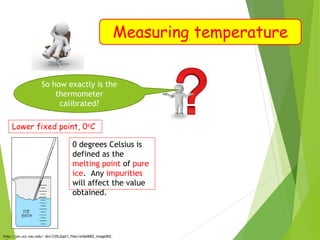
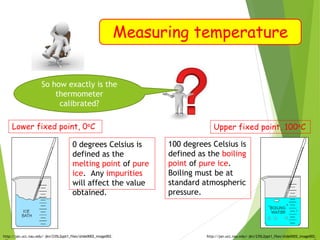
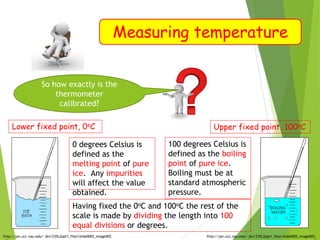
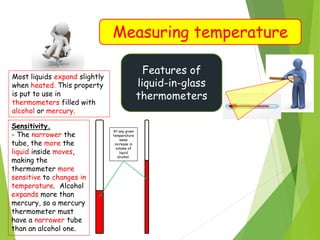
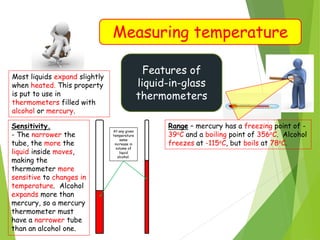
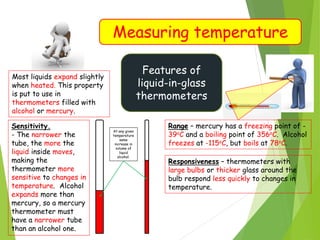
- Measurement of temperature using properties that vary with temperature, such as liquid-in-glass thermometers which are calibrated using the fixed points of 0°C and 100°C.
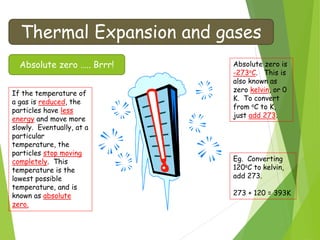
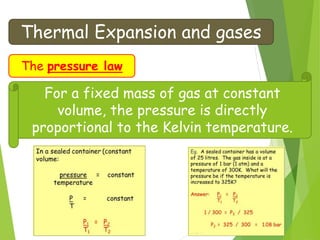
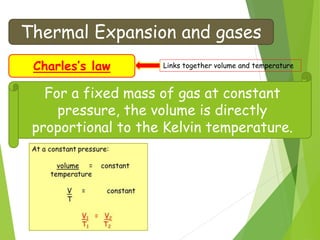
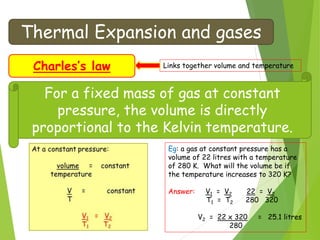
- The relationship between gas pressure and temperature defined by the gas laws, where pressure and volume directly depend on temperature when other variables are held constant.