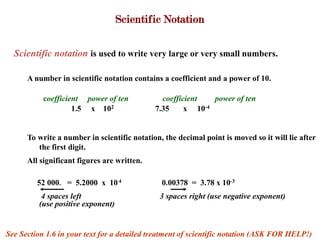
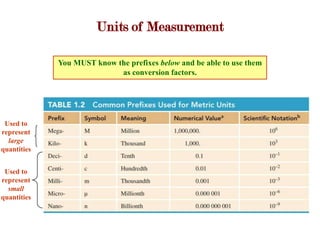
1. Scientific notation is used to write very large or small numbers and involves moving the decimal point so the first non-zero digit is to the left of the decimal, with the power of 10 indicating the number of places the decimal was moved. Units of measurement and prefixes are used to represent large and small quantities.
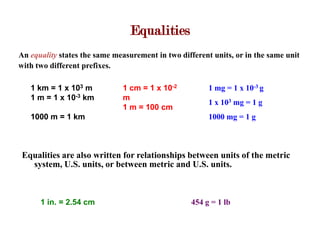
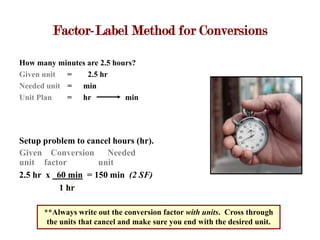
2. Equalities show equivalent measurements between units and can be used to derive conversion factors. The factor-label method involves setting up a ratio between the given and desired units, with the unit in the numerator cancelling the unit in the denominator to give the conversion in the desired unit.
3. Examples are provided for measuring temperatures on thermometers, converting between units like hours to minutes using conversion factors