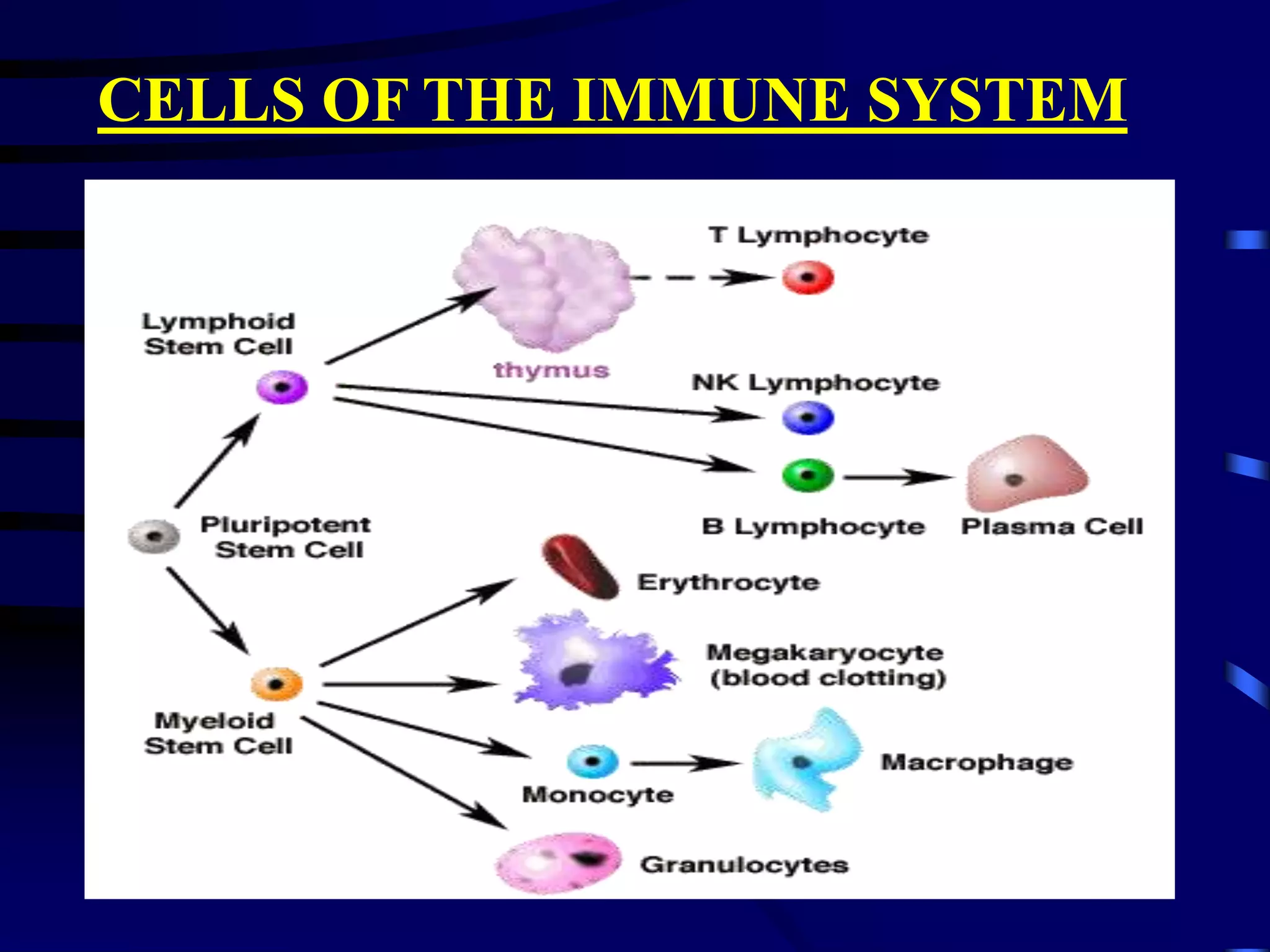
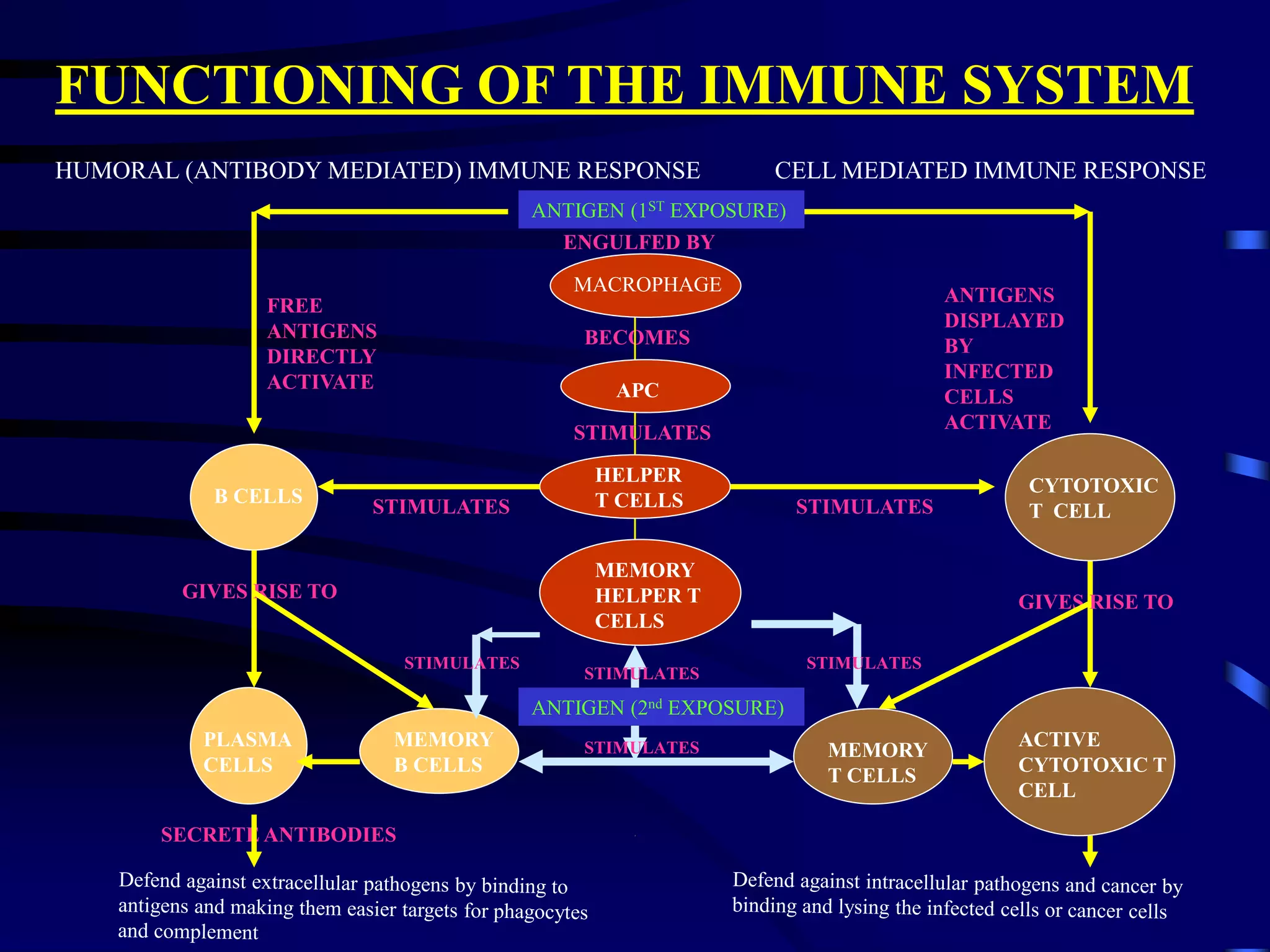
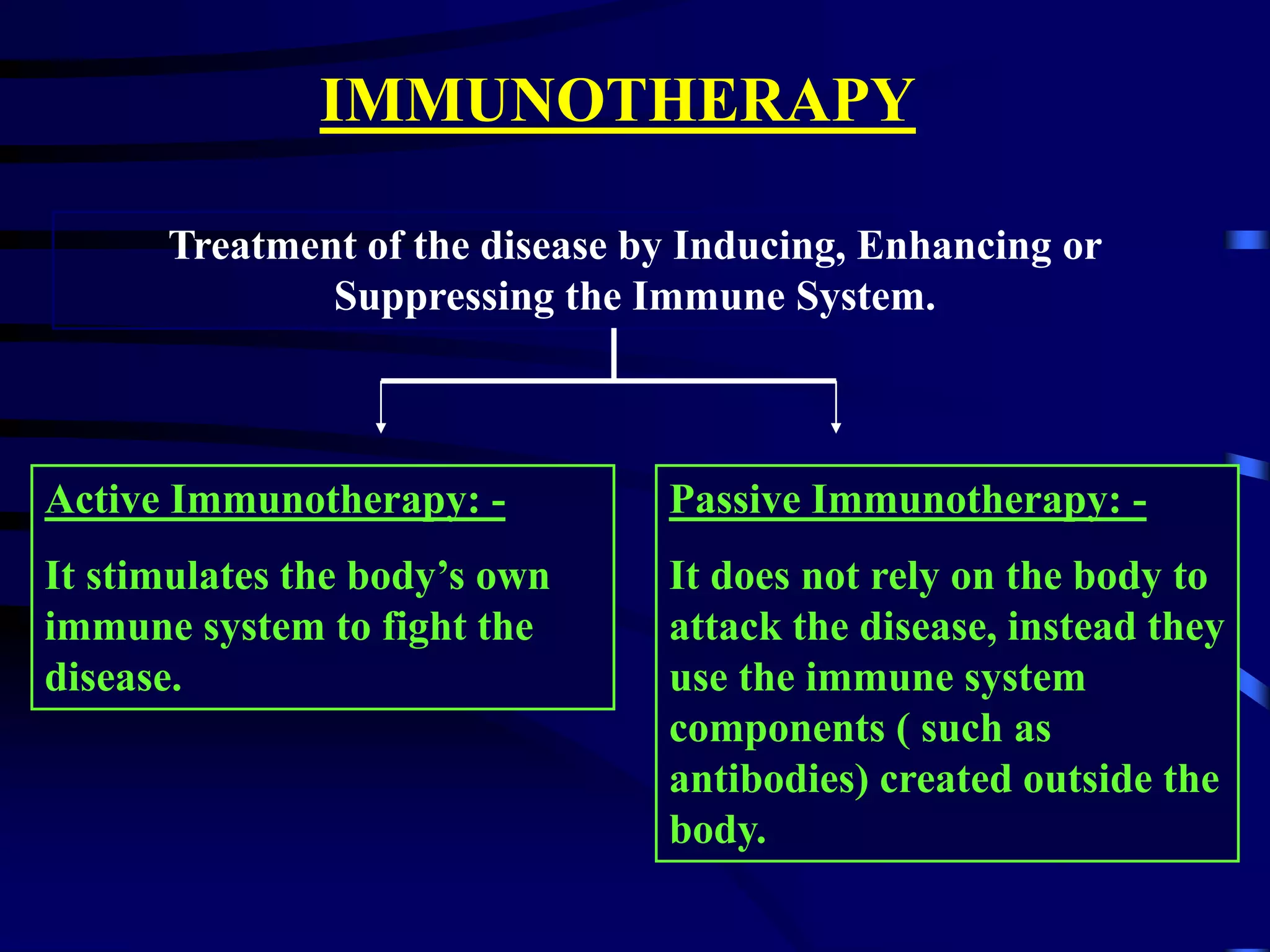
This document provides an overview of monoclonal antibodies and their importance. It begins with definitions of the immune system and its components. It then discusses the anatomy and cells of the immune system, and how it functions through both humoral and cell-mediated responses. The document explains the production of monoclonal antibodies using hybridoma technology. It lists FDA-approved monoclonal antibodies and their applications in diagnosis, treatment of diseases like cancer and infection. The market for monoclonal antibodies is projected to reach $26 billion by the end of the decade due to their role in driving the biotechnology industry and success in developing new therapies.