Embed presentation
Download to read offline
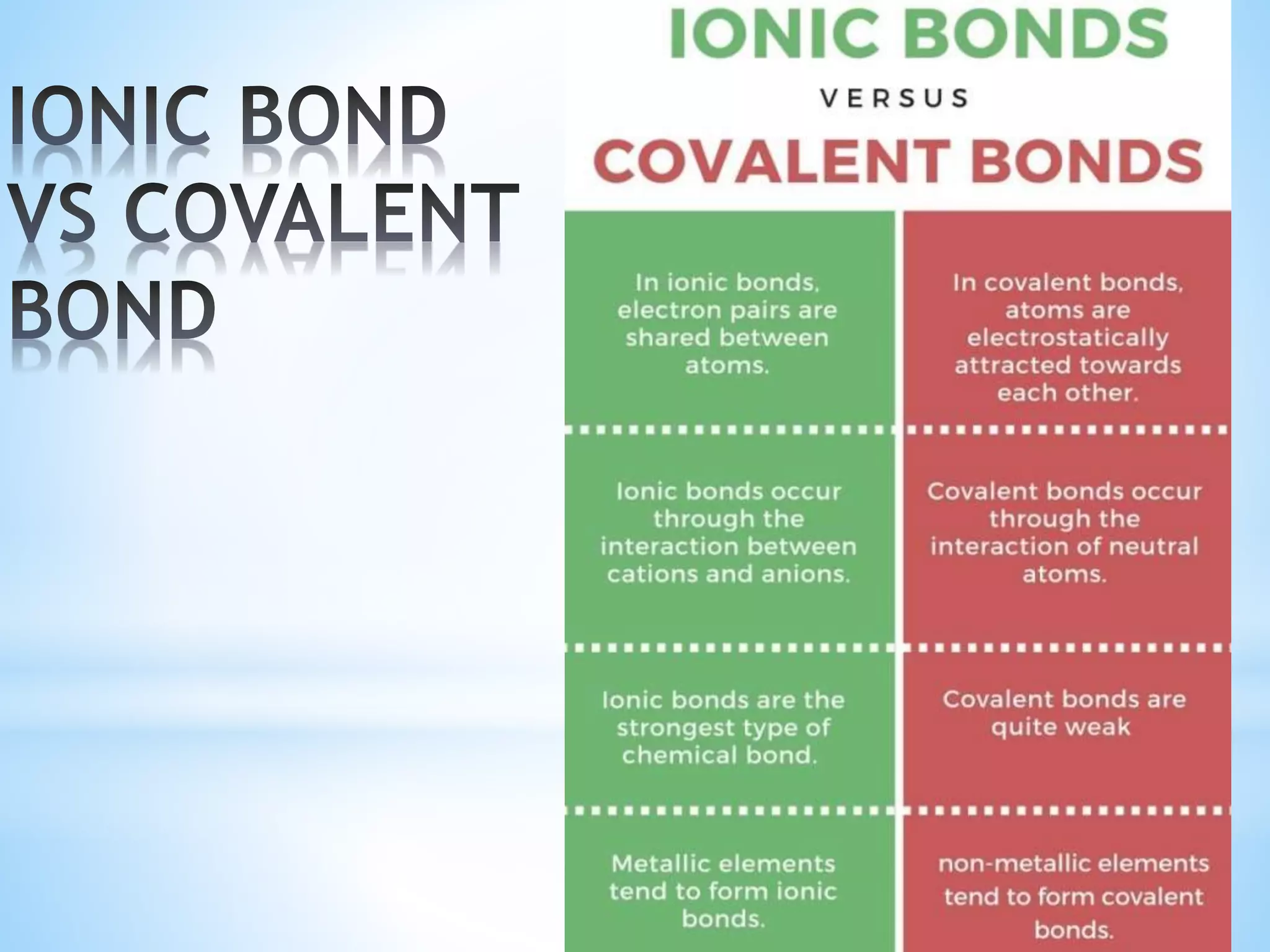

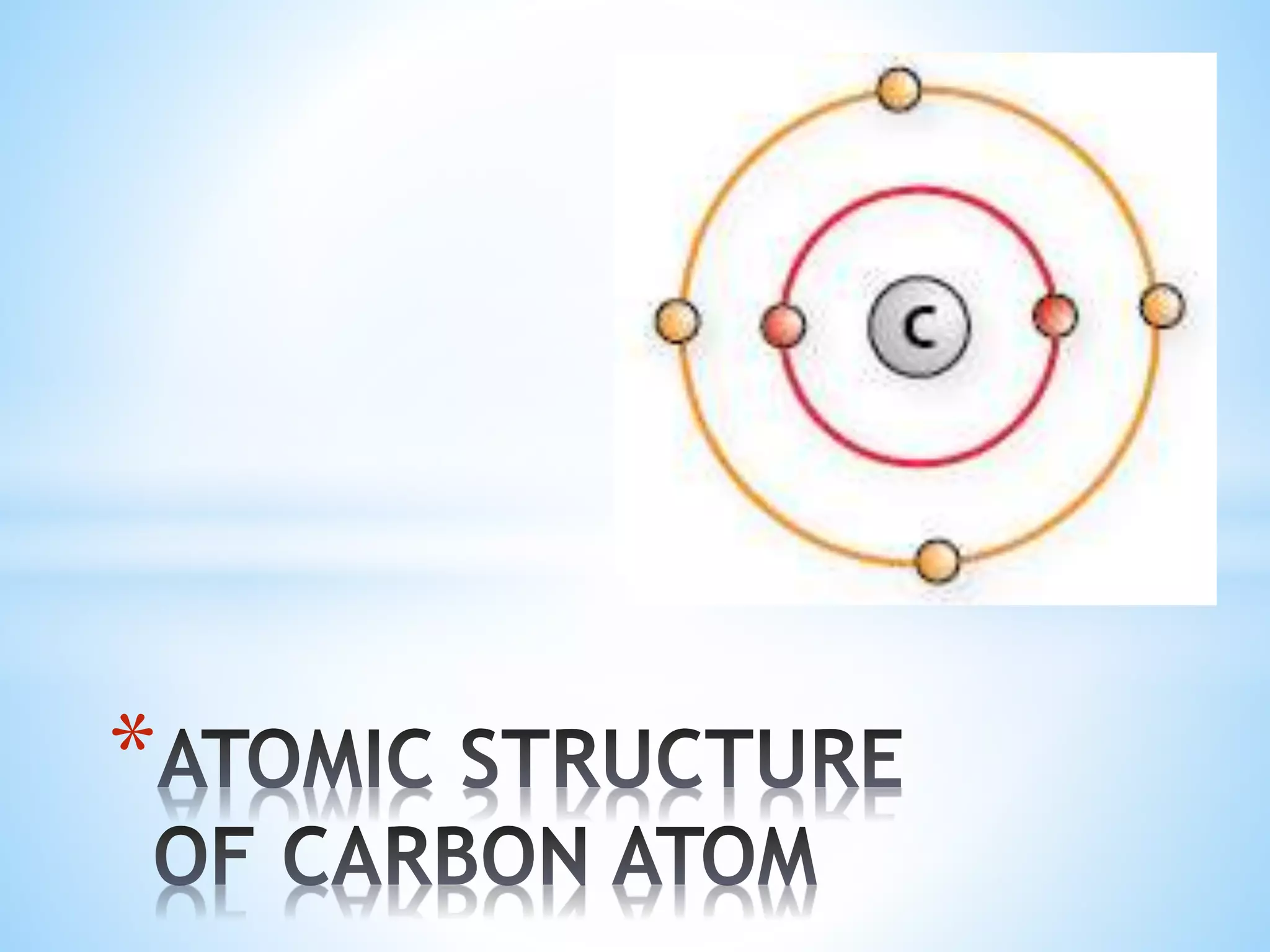


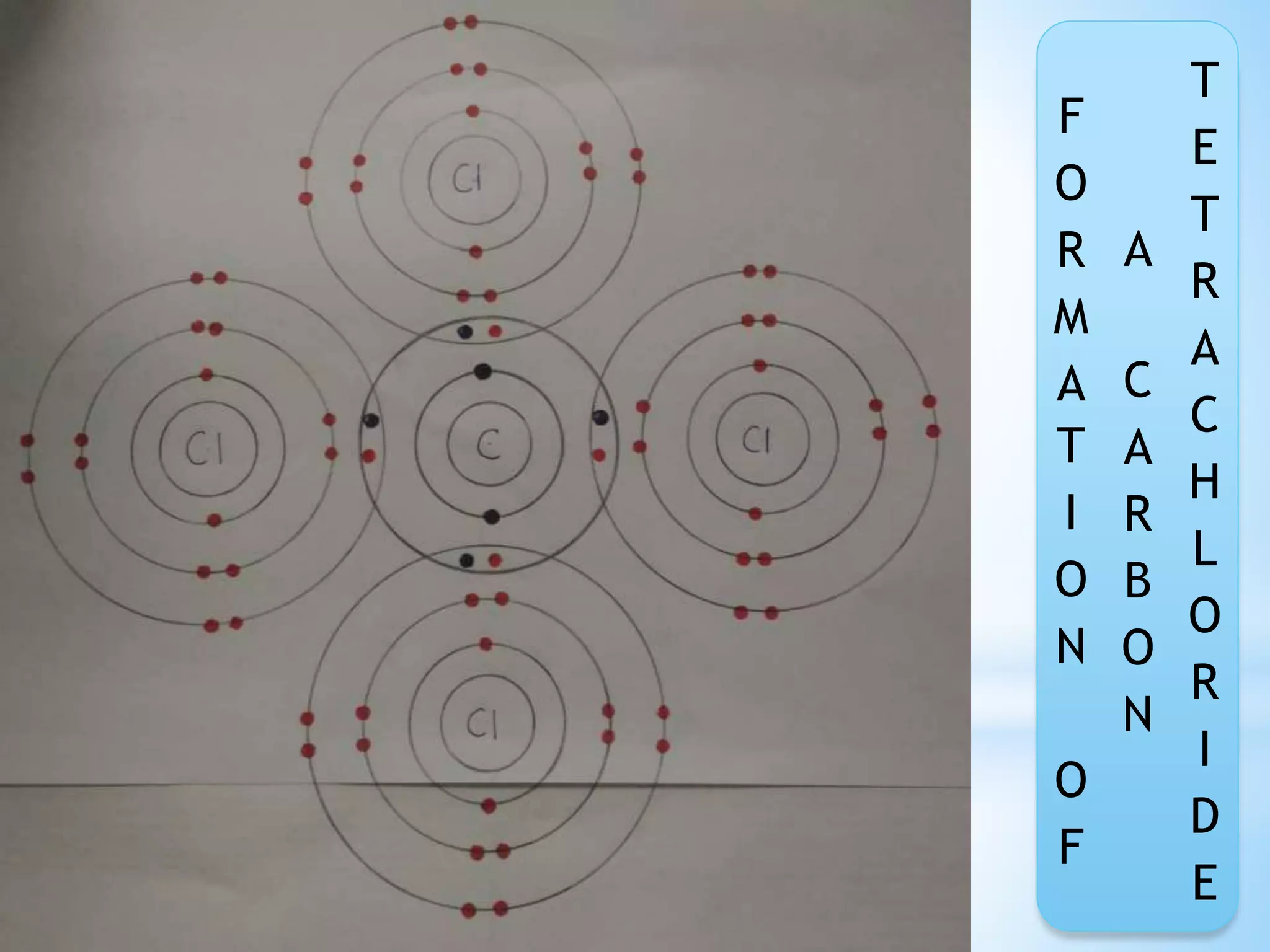



Carbon tetrachloride is formed when one carbon atom shares its four valence electrons with four chloride ions to achieve stable electron configurations. Each chloride ion contributes one electron to be shared, and the carbon atom contributes four electrons. This sharing of electrons forms four single covalent bonds between the carbon atom and the chloride ions, creating the CCl4 molecule with a stable Lewis structure.







