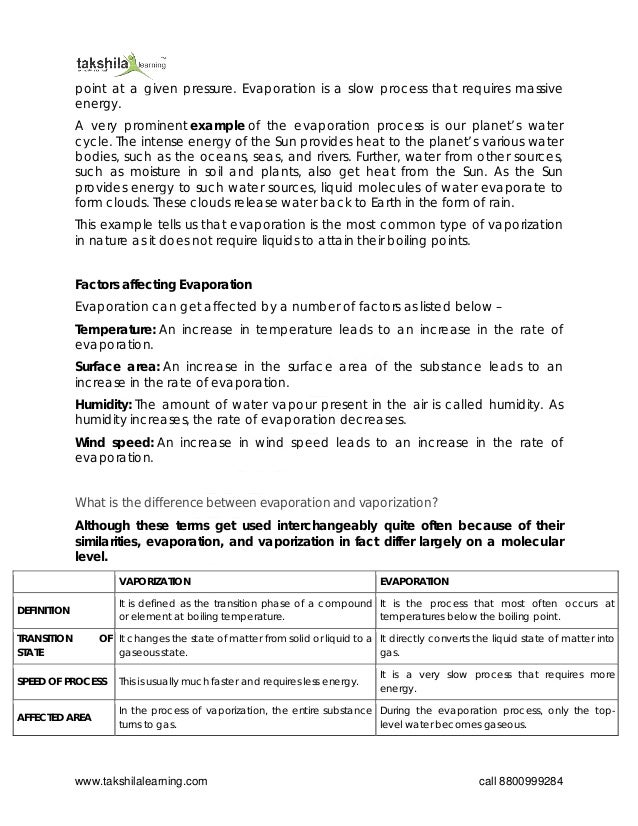
The document discusses the concepts of evaporation and vaporization, explaining that while both processes involve the transition from liquid or solid to gas, they occur under different conditions and at different rates. Evaporation is a type of vaporization that occurs at temperatures below boiling points and is a slower process influenced by factors such as temperature, surface area, humidity, and wind speed. The document concludes by highlighting the main differences between the two processes on a molecular level and emphasizes that both are physical changes with no alteration in chemical composition.