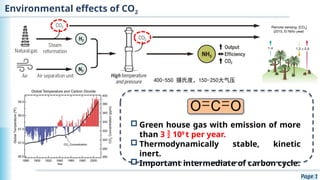
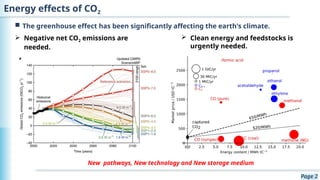
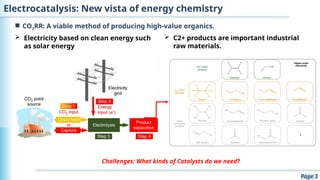
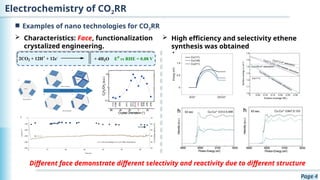
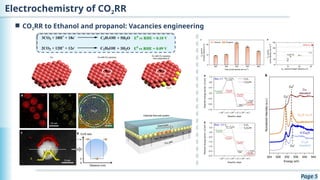
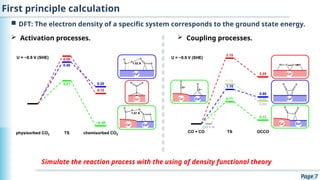
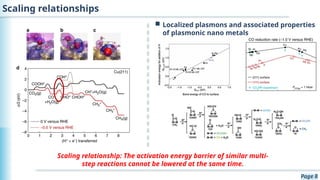
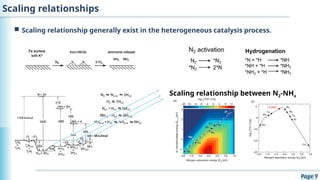
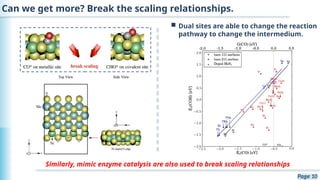
The document discusses the environmental impact of CO2 emissions and the urgent need for negative net CO2 emissions through clean energy and innovative technologies such as electrocatalysis. It highlights CO2 reduction reactions (CO2RR) as a promising method for producing valuable organic compounds and explores challenges in catalyst efficiency and selectivity. Additionally, it addresses potential future advancements in catalyst screening using machine learning.