This document provides conceptual problems and questions about solids and the theory of conduction. It includes:
1. Questions about how energy is lost by electrons colliding with ions and how this appears as Joule heat.
2. Questions about the resistivity of brass and copper at low temperatures being due to residual resistance from impurities.
3. Problems involving calculating contact potentials between different metals using their work functions.
4. Additional conceptual questions about properties of conductors, insulators, semiconductors and how doping affects conduction.




![Chapter 38
1266
( )
( ) ( )
[ ]
3
3
2
3
6
3
9
23
g/cm
07
.
2
cm
10
m
1
g/m
10
07
.
2
m
10
257
.
0
2
mol
particles/
10
02
.
6
g/mol
4
.
42
4
=
⎟
⎟
⎠
⎞
⎜
⎜
⎝
⎛
×
×
=
×
×
=
−
ρ
*18 •
Picture the Problem We can solve Equation 38-6 for n.
Equation 38-6 is:
( ) ⎟
⎠
⎞
⎜
⎝
⎛
−
−
=
n
r
ke
r
U
1
1
0
2
0 α
and
( ) ⎟
⎠
⎞
⎜
⎝
⎛
−
=
n
r
ke
r
U
1
1
0
2
0 α
Solve for n to obtain:
( )
2
0
0
1
1
ke
r
r
U
n
α
−
=
Substitute numerical values and evaluate n:
( ) ( )
( )( )
4.64
nm
eV
44
.
1
7476
.
1
nm
257
.
0
kJ/mol
47
.
96
pair
eV/ion
1
kJ/mol
741
1
1
=
⋅
⎟
⎟
⎠
⎞
⎜
⎜
⎝
⎛
−
=
n
19 ••
Picture the Problem We can substitute numerical values in Equation 38-6 to evaluate
U(ro) for n = 8 and n = 10.
(a) Equation 38-6 is:
( ) ⎟
⎠
⎞
⎜
⎝
⎛
−
−
=
n
r
ke
r
U
1
1
0
2
0 α
Substitute numerical values and evaluate U(r0):
( ) ( )( )( )
eV
6
.
10
J
10
1.60
1eV
8
1
1
m
10
0.208
J
10
1.60
/C
m
N
10
8.99
1.7476
19
9
2
19
2
2
9
o
−
=
⎟
⎠
⎞
⎜
⎝
⎛
×
⎟
⎠
⎞
⎜
⎝
⎛
−
×
×
⋅
×
−
= −
−
−
r
U
(b) The fractional change is given by: ( )
( )
1
8
10
8
8
10
0
0
−
=
−
=
∆
=
=
=
=
=
n
n
n
n
n
U
U
U
U
U
r
U
r
U](https://image.slidesharecdn.com/ismchapter38-220316050800/85/capt-38-6-320.jpg)







![Chapter 38
1274
33 ••
Picture the Problem We can follow the procedure given in the problem statement to
show that 3
5
F
5
2 −
=
= CV
V
NE
P and
V
NE
P
B
3
2
3
5 F
=
= .
(a) From Problem 32 we have: av
3
2
NE
PV =
Because :
F
5
3
av E
E =
F
F
5
2
5
3
3
2
E
V
N
E
V
N
P ⎟
⎠
⎞
⎜
⎝
⎛
=
⎟
⎠
⎞
⎜
⎝
⎛
⎟
⎠
⎞
⎜
⎝
⎛
= (1)
The Fermi energy is given by: 3
2
3
2
e
2
3
2
e
2
F
3
8
3
8
−
⎟
⎠
⎞
⎜
⎝
⎛
=
⎟
⎠
⎞
⎜
⎝
⎛
= V
N
m
h
V
N
m
h
E
π
π
Substitute to obtain:
3
5
3
5
3
2
e
2
3
5
3
2
3
2
e
2
3
20
3
8
5
2
−
−
−
=
⎟
⎠
⎞
⎜
⎝
⎛
=
⎟
⎠
⎞
⎜
⎝
⎛
⎟
⎠
⎞
⎜
⎝
⎛
=
CV
V
m
h
N
V
N
m
h
V
N
P
π
π
where
3
2
e
2
3
5
3
20
⎟
⎠
⎞
⎜
⎝
⎛
=
π
m
h
N
C is a constant.
(b) The bulk modulus is given by:
[ ]
P
CV
V
CV
CV
dV
d
V
dV
dP
V
B
3
5
3
5
3
5 3
5
3
8
3
5
=
=
⎟
⎠
⎞
⎜
⎝
⎛
−
−
=
−
=
−
=
−
−
−
Substitute for P from equation (1) to
obtain: F
F
3
2
5
2
3
5
E
V
N
E
V
N
B ⎟
⎠
⎞
⎜
⎝
⎛
=
⎥
⎦
⎤
⎢
⎣
⎡
⎟
⎠
⎞
⎜
⎝
⎛
=
(c) Substitute numerical values and evaluate B for copper:
( )( )( ) 2
9
19
3
22
N/m
10
6
.
63
J/eV
10
60
.
1
eV
04
.
7
cm
electrons/
10
47
.
8
3
2
×
=
×
×
= −
B
From Table 13-2: BCu = 140 GN/m2](https://image.slidesharecdn.com/ismchapter38-220316050800/85/capt-38-14-320.jpg)








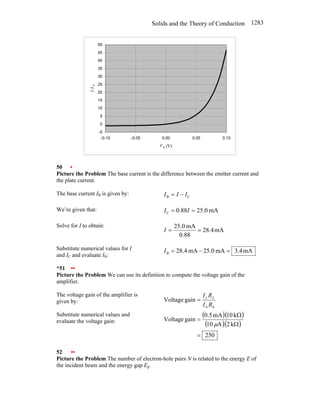

![Solids and the Theory of Conduction 1285
Substitute for I in equation (1) and
simplify:
0
b
0
b
eI
kT
kT
eV
I
V
R =
=
Substitute numerical values and
evaluate R:
( )( )
( )( )
Ω
=
×
×
= −
−
−
M
0
.
25
A
10
C
10
60
.
1
eV
/
J
10
60
.
1
eV
025
.
0
9
19
19
R
(b) Substitute equation (2) in
equation (1) to obtain:
( )
1
b
0
b
−
= kT
eV
e
I
V
R (3)
Evaluate
kT
eVb
for Vb = − 0.5 V:
( )( )
( )( )
8
.
19
K
293
J/K
10
1.38
V
5
.
0
C
10
60
.
1
23
19
b
−
=
×
−
×
= −
−
kT
eV
Evaluate equation (3) for
Vb = − 0.5 V: ( )( ) Ω
=
−
−
= −
−
M
500
1
A
10
V
5
.
0
8
.
19
9
e
R
(c) Evaluate
kT
eVb
for
Vb = + 0.5 V:
( )( )
( )( )
8
.
19
K
293
J/K
10
1.38
V
5
.
0
C
10
60
.
1
23
19
b
=
×
×
= −
−
kT
eV
Evaluate equation (3) for
Vb = +0.5 V: ( )( ) Ω
=
−
= −
26
.
1
1
A
10
V
5
.
0
8
.
19
9
e
R
(d) Evaluate Rac = dV/dI to obtain:
( )
[ ]
kT
eV
kT
eV
kT
eV
e
eI
kT
e
kT
eI
e
I
dV
d
dV
dI
dI
dV
R
b
b
b
0
1
0
1
0
1
ac
1
−
−
−
−
=
⎭
⎬
⎫
⎩
⎨
⎧
=
⎭
⎬
⎫
⎩
⎨
⎧
−
=
⎟
⎠
⎞
⎜
⎝
⎛
=
=
Substitute numerical values and
evaluate Rac:
( ) Ω
=
Ω
= −
0629
.
0
M
25 8
.
19
ac e
R
55 ••
Picture the Problem We can use the Hall-effect equation to find the concentration of
charge carriers in the slab of silicon. We can determine the semiconductor type by
determining the directions of the magnetic and electric fields.
Use the expression for the Hall-
effect voltage to relate the
concentration of charge carriers n to
nte
IB
V =
H](https://image.slidesharecdn.com/ismchapter38-220316050800/85/capt-38-25-320.jpg)








![Chapter 38
1294
( ) ( )
1
1
F
+
= − kT
E
E
e
E
f
The dimensionless exponent in the
Fermi factor is:
1
g
2
1
F
>>
−
=
−
kT
E
E
kT
E
E
and
1
exp g
2
1
>>
⎟
⎟
⎠
⎞
⎜
⎜
⎝
⎛ −
kT
E
E
Hence:
( ) ( )
kT
E
kT
E
kT
E
E
e
e
e
E
f −
−
≈
=
2
g
g
2
1
1
Substitute in equation (1) and
simplify to obtain:
( ) ( ) kT
E
kT
E
F e
E
e
NE
E
n −
−
= 2
1
2
2
3
2
3 g
There is an additional temperature dependence that arises from the fact that EF depends
on T. At room temperature, exp[(E − Eg/2)/kT] ≥ exp(0.35 eV/0.0259 eV) = 7.4×105
, so
the approximation leading to the Boltzmann distribution is justified.
*70 •••
Picture the Problem We can follow the step-by-step procedure outlined in the problem
statement to obtain the indicated results.
(a) The Fermi factor is: ( ) ( )
1
1
1
1
1
1
F
F
+
=
+
=
+
= −
−
kT
E
kT
E
kT
E
kT
E
E
Ce
e
e
e
E
f
provided
kT
E
e
C F
−
=
(b) If C >> e−E/kT
:
( ) kT
E
kT
E
kT
E
Ae
Ce
Ce
E
f −
=
≈
+
=
1
1
1
where A = 1/C
(c) The energy distribution function
is:
( ) ( ) ( )
E
f
dE
E
g
dE
E
n =
where
( ) 2
1
3
2
3
e
2
8
E
h
V
m
E
g
π
=
Substitute for g(E)dE and f(E) in the
expression for N to obtain: ∫
∞
−
=
0
2
1
3
2
3
e
2
8
dE
e
E
h
V
m
A
N kT
E
π](https://image.slidesharecdn.com/ismchapter38-220316050800/85/capt-38-34-320.jpg)
![Solids and the Theory of Conduction 1295
The definite integral has the value: ( ) π
2
2
3
0
2
1 kT
dE
e
E kT
E
=
∫
∞
−
Substitute to obtain: ( ) π
π
2
2
8
2
3
3
2
3
e kT
h
V
m
A
N =
Solve for A:
( ) 2
3
2
3
e
2
3
3
1
8
2
kT
V
N
m
h
A ⎟
⎠
⎞
⎜
⎝
⎛
=
π
(d) Evaluate A at T = 300 K:
( )
( ) ( )( )
[ ]
10
4
K
300
J/K
10
38
.
1
kg
10
11
.
9
8
s
J
10
63
.
6
2 26
2
3
23
2
3
31
2
3
3
34
n
n
A −
−
−
−
×
≈
×
×
⋅
×
=
π
where the units are SI.
re.
temperatu
higher the
e
greater th
be
may
ion
concentrat
electron
the
,
on
depends
Because
ion.
concentrat
electron
valence
the
of
millionth
one
about
or
,
m
10
than
less
be
should
re,
temperatu
room
at
1
that
condition
e
satisfy th
To
.
m
10
about
typically
is
ion
concentrat
electron
valence
The
2
3
3
23
3
39
−
−
−
<<
T
A
n
A
(e)
.
applicable
is
ion
approximat
classical
the
),
(
in
criterion
the
to
according
So,
.
m
10
cm
10 3
23
3
17
d
−
−
=
71 •••
Picture the Problem We can approximate the separation of electrons in the gas by
(V/N)1/3
and use the for A from Problem 70 and de Broglie’s equation to express the
separation d of electrons in terms of the de Broglie wavelength λ and the constant A.
The separation d of electrons is
approximately:
3
1
⎟
⎠
⎞
⎜
⎝
⎛
=
N
V
d
From Problem 70:
( ) 3
1
2
1
2
1
e
2
1
3
1
6
1
3
1
1
8
2
A
kT
m
h
N
V
π
=
⎟
⎠
⎞
⎜
⎝
⎛
Substitute to obtain:
( )
3
1
e
2
1
6
1
3
1
2
1
2
1
e
2
1
3
1
6
1
2
1
2
1
8
2
A
kT
m
h
A
kT
m
h
d
π
π
=
=](https://image.slidesharecdn.com/ismchapter38-220316050800/85/capt-38-35-320.jpg)




