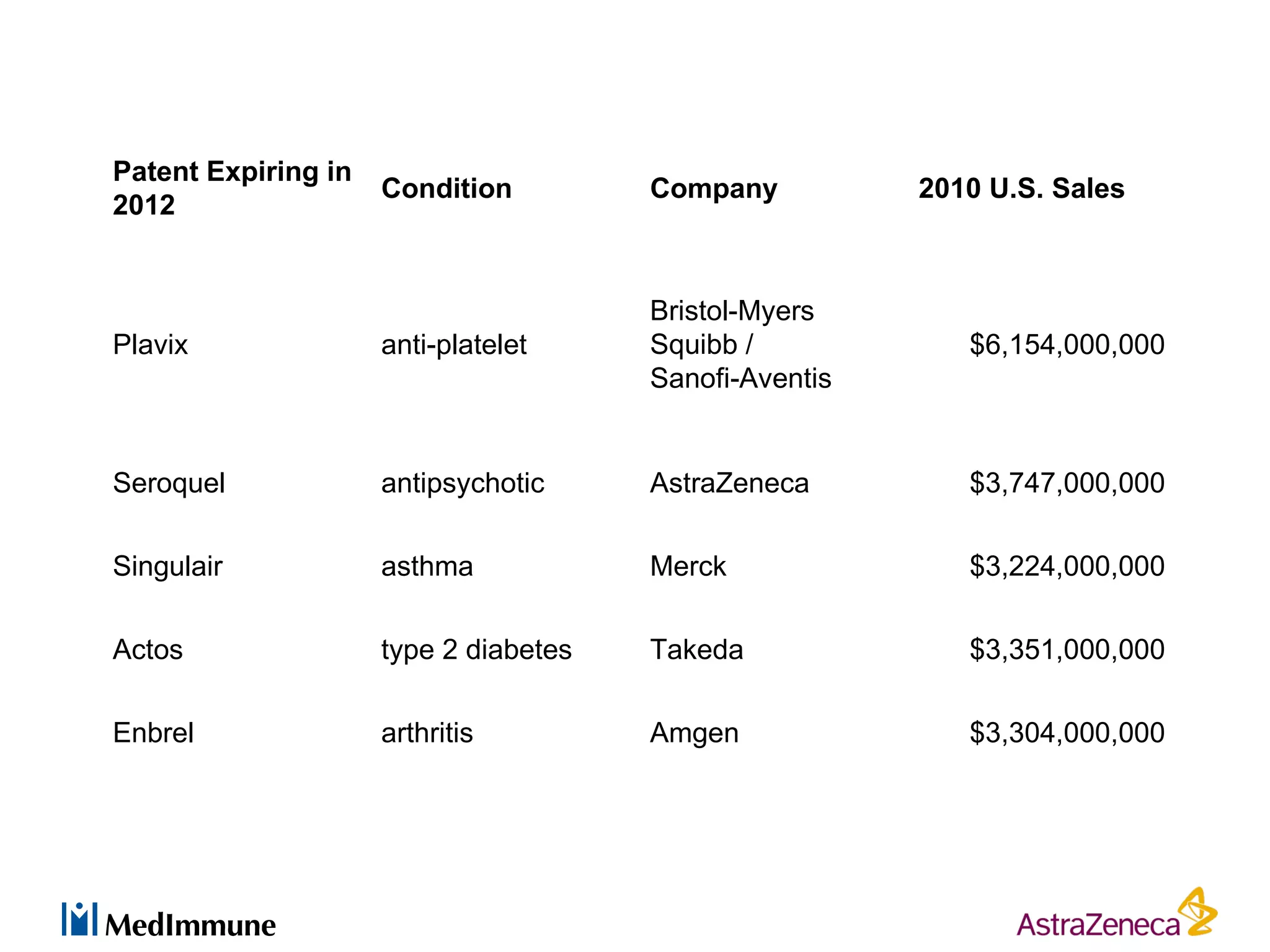
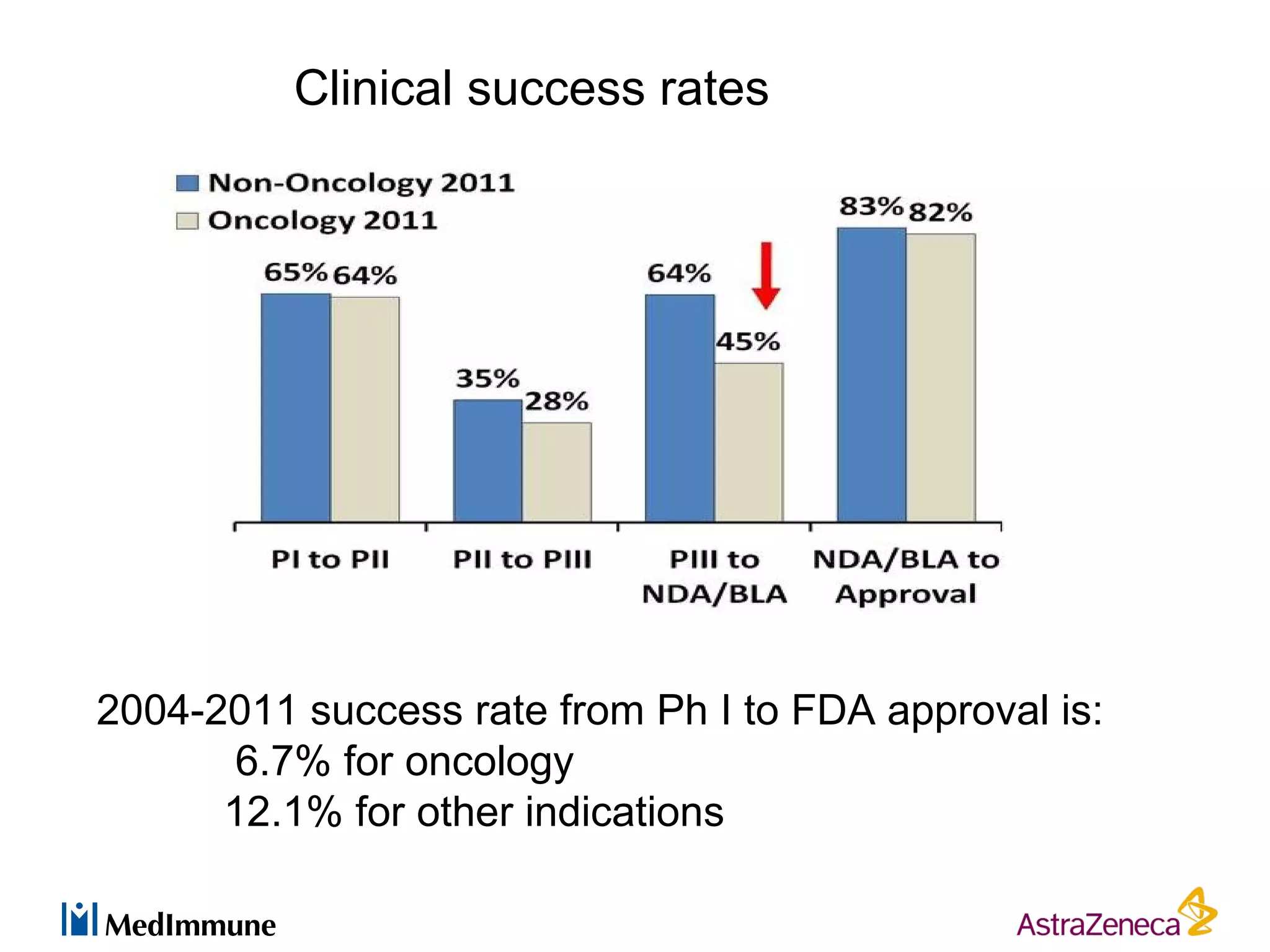
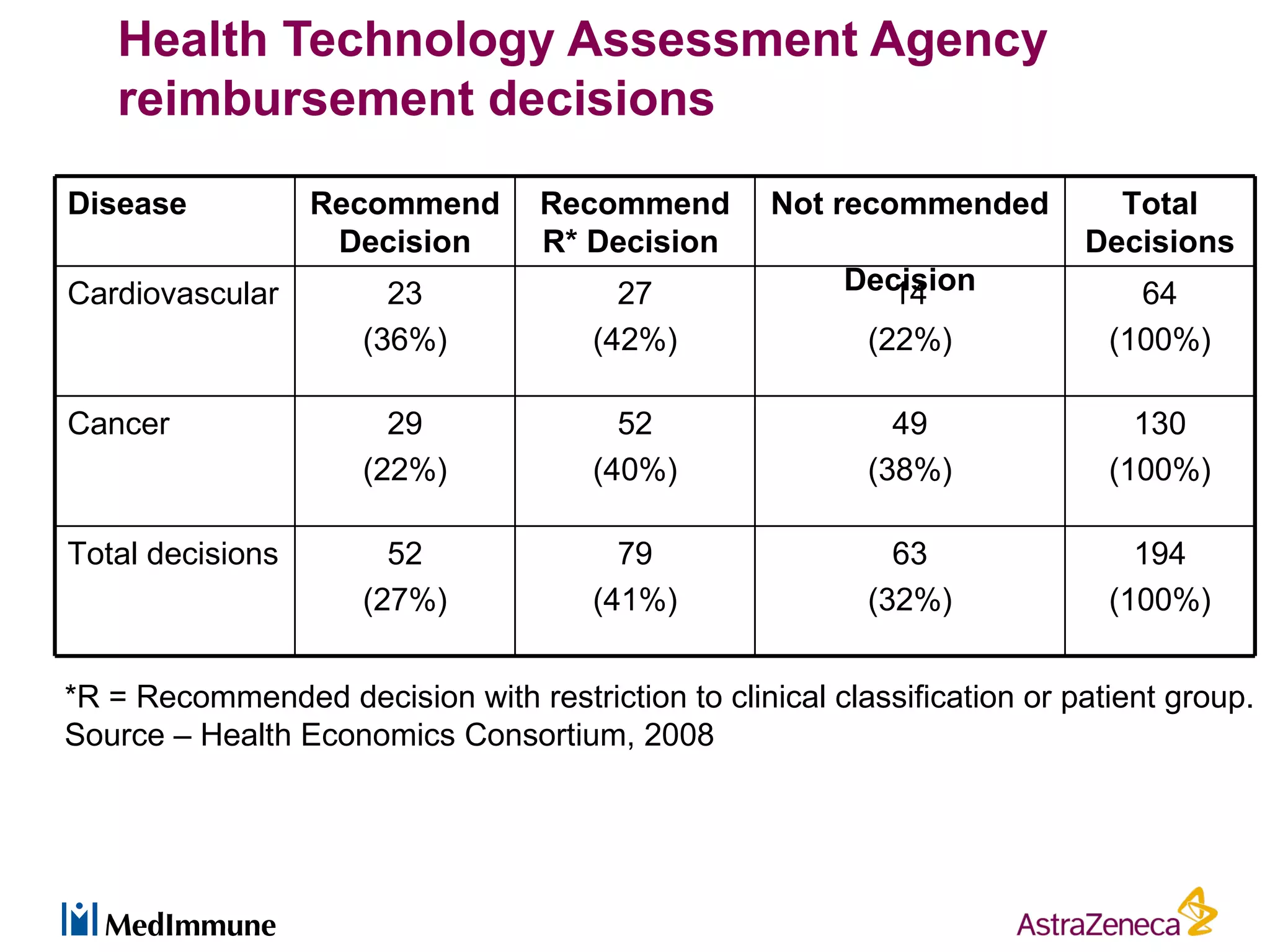
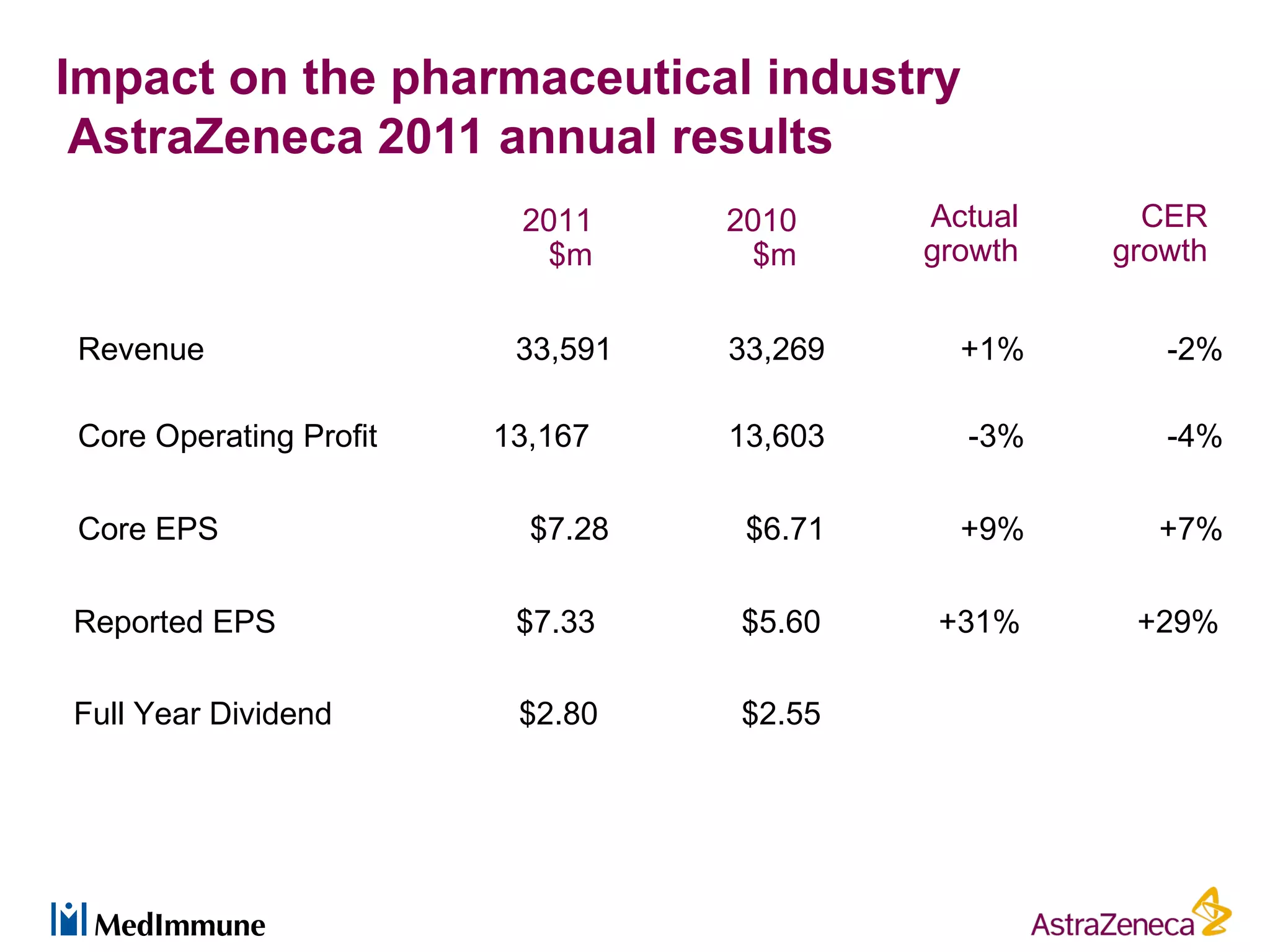
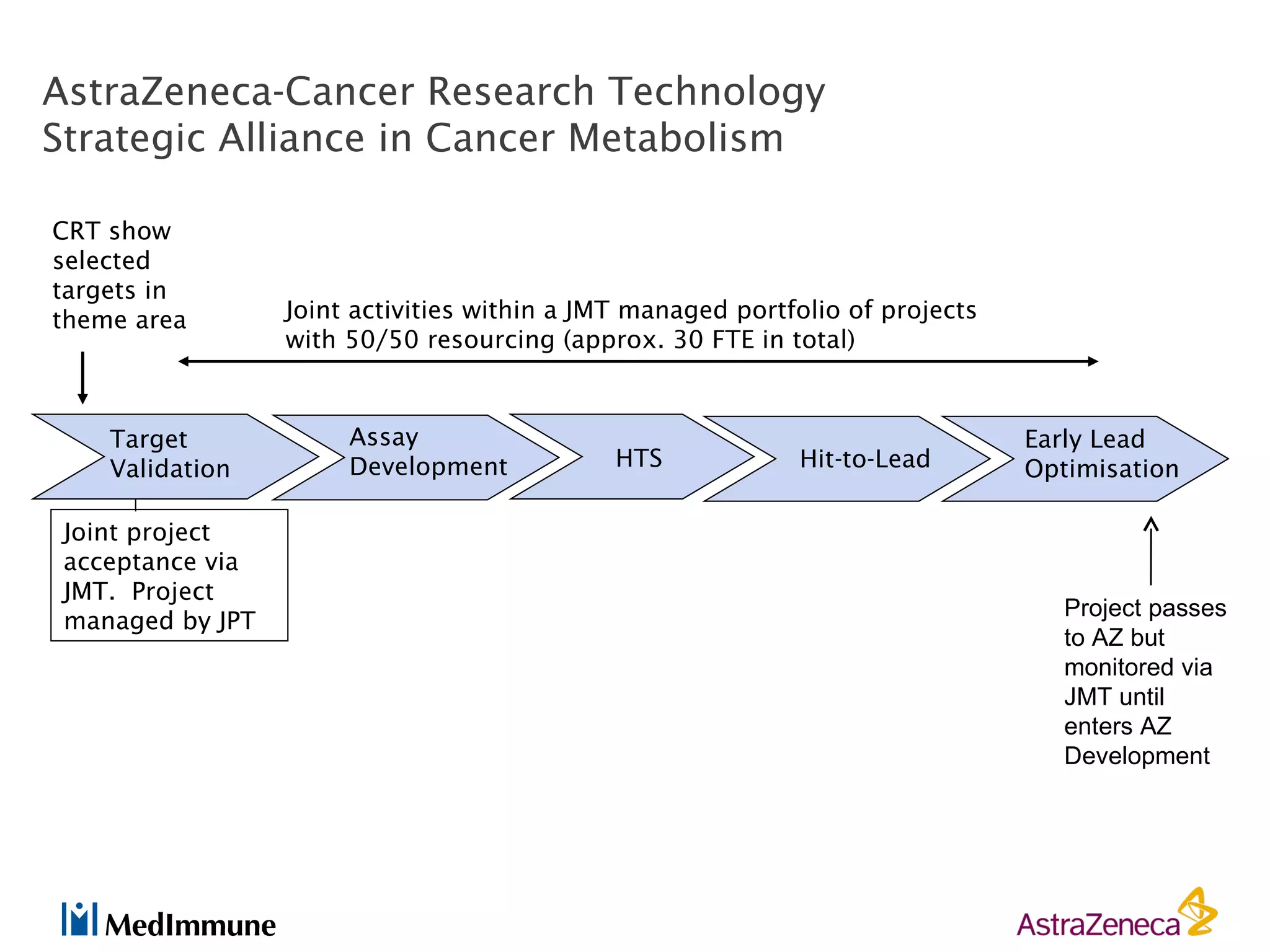
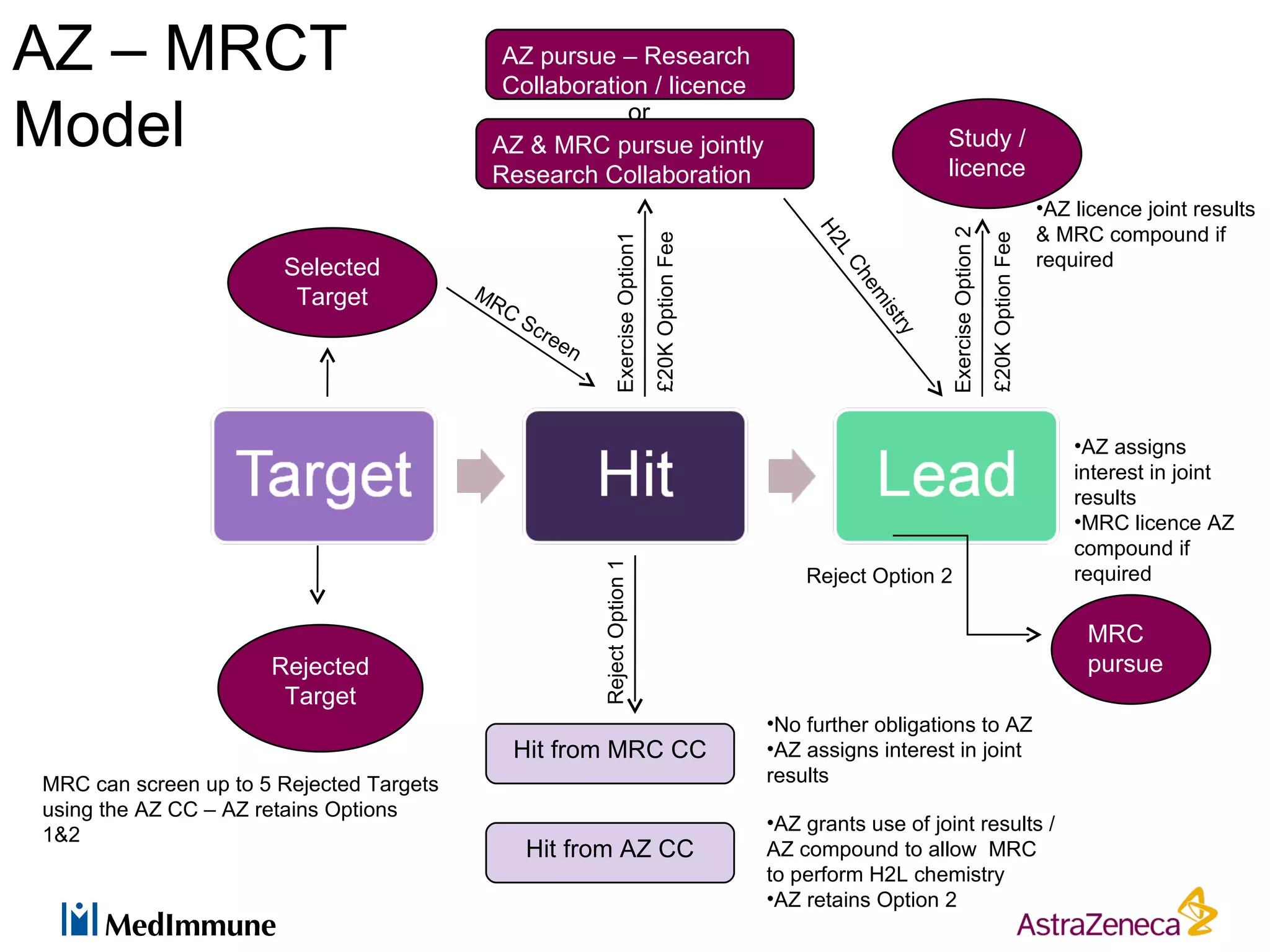
The pharmaceutical industry faces significant challenges in sustaining drug discovery and innovation due to factors such as patent expiries, regulatory hurdles, pricing pressures, and low clinical success rates. In response, companies are focusing resources on late-stage pipelines and reducing early-stage research. However, this threatens long-term sustainability. Creative collaboration between industry and academia, where resources and risks/rewards are shared, shows promise as a model to help secure ongoing innovation through drug discovery. AstraZeneca has formed a strategic alliance with Cancer Research Technology to jointly work on projects in cancer metabolism as an example of this collaborative approach.