1) The study developed a low-cost screening system using freely available ImageJ software to analyze video recordings of the swimming behavior and kinetics of different life stages of the sea louse Caligus rogercresseyi exposed to potential drug compounds.
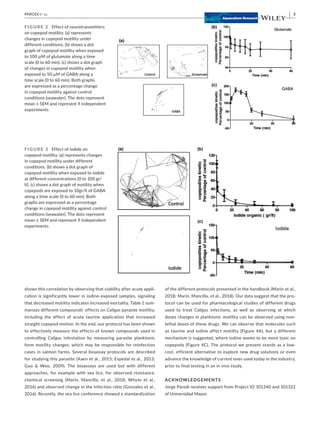
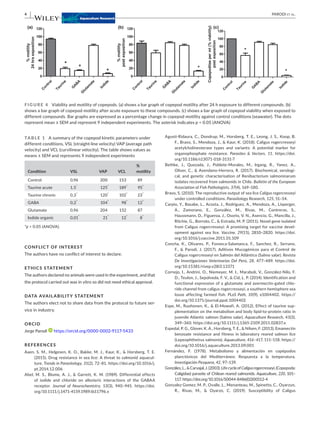
2) Results showed that taurine, glutamate, GABA, and iodine all impacted the motility of C. rogercresseyi planktonic forms in a time- and dose-dependent manner, with iodine having the strongest inhibiting effects.
3) The system provides a way to efficiently and inexpensively study the pharmacological effects of potential treatments on C. rogercresseyi before conducting more complex in vitro