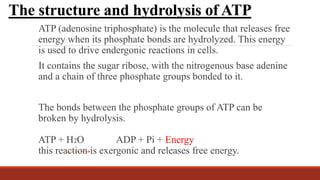
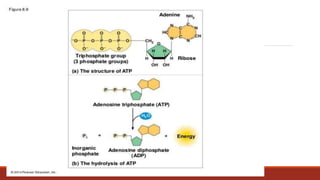
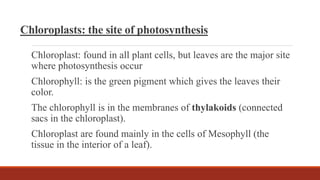
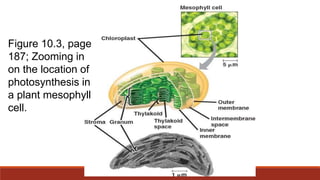
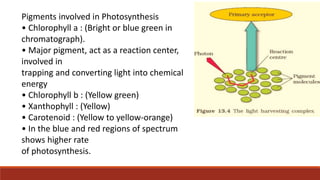
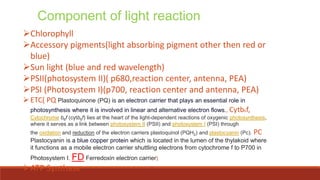
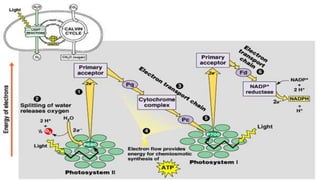
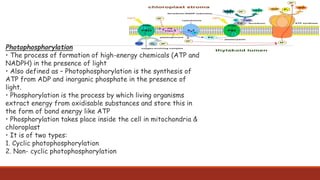
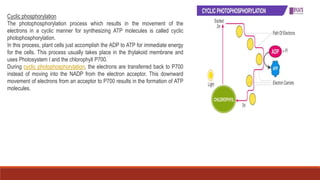
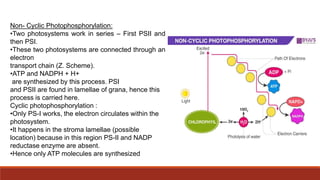
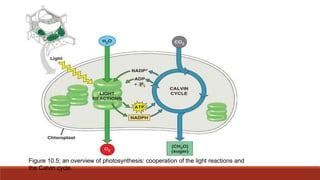
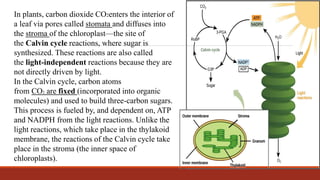
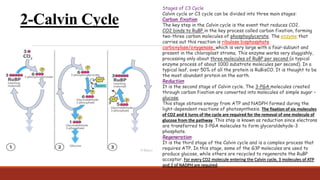
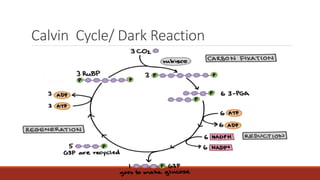
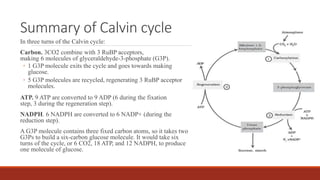
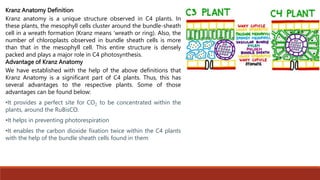
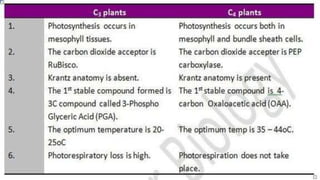
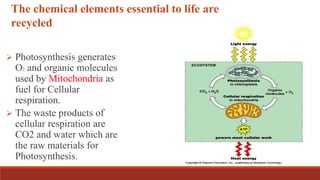
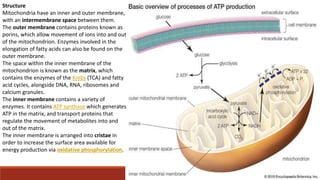
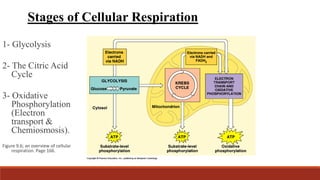
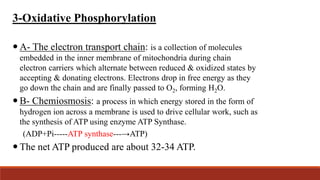
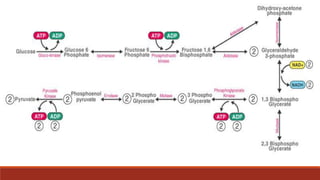
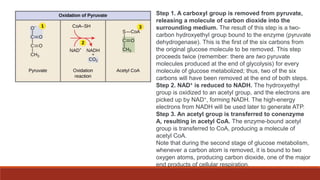
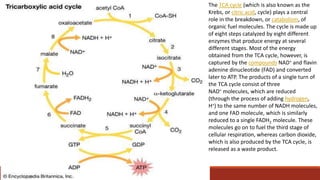
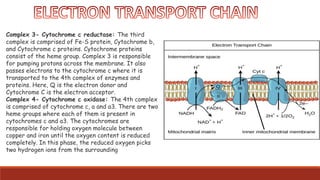
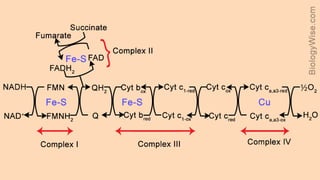
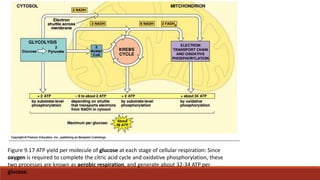
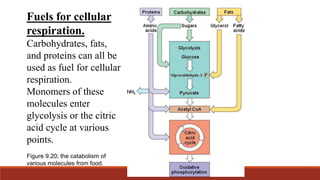
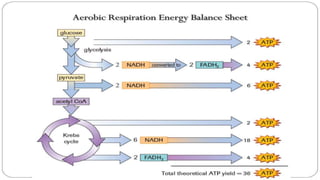
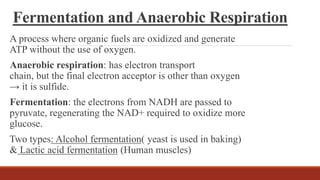
The document discusses bioenergetics, which focuses on how cells transform energy through processes like cellular respiration and photosynthesis. These bioenergetic processes are essential to life. ATP (adenosine triphosphate) is the molecule that cells use to store and transport energy. ATP is produced through catabolic pathways that break down molecules and through phosphorylation, either substrate-level or oxidative phosphorylation during cellular respiration. Photosynthesis uses light energy to produce sugars and oxygen from carbon dioxide and water. This involves two stages - the light-dependent reactions where ATP and NADPH are produced, and the Calvin cycle where sugars are formed.