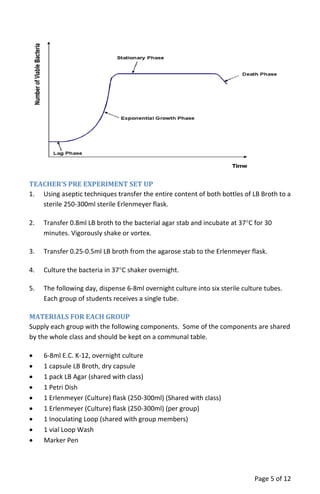
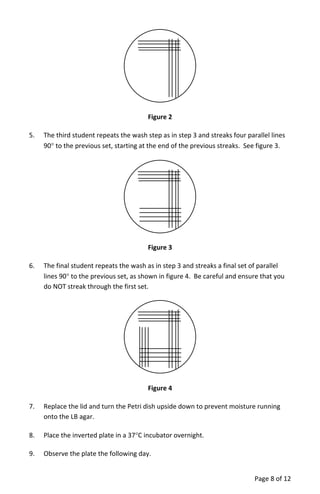
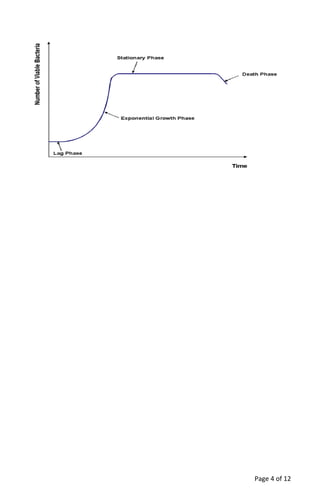
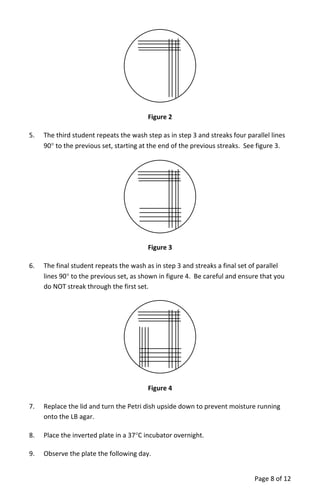
The document provides a teacher's guide for conducting a bacterial culture and growth experiment using a kit designed for 24 students. It outlines the objectives, materials needed, special handling instructions, and detailed procedures for preparing media, inoculating cultures, and analyzing results regarding bacterial growth phases. The activity aims to teach students aseptic techniques and the biological principles underlying bacterial growth in liquid and solid cultures.