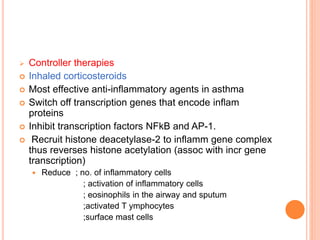
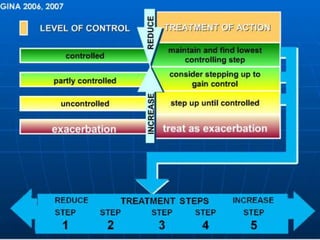
This document defines bronchial asthma and discusses its epidemiology, etiology, pathology, clinical features, diagnosis, classification of severity, and treatment. Some key points:
- Asthma is a chronic inflammatory disorder characterized by airway hyperresponsiveness leading to reversible airflow obstruction. It affects 300 million people globally.
- Both genetic and environmental factors contribute to asthma development, including atopy, air pollution, allergens, and occupational sensitizers.
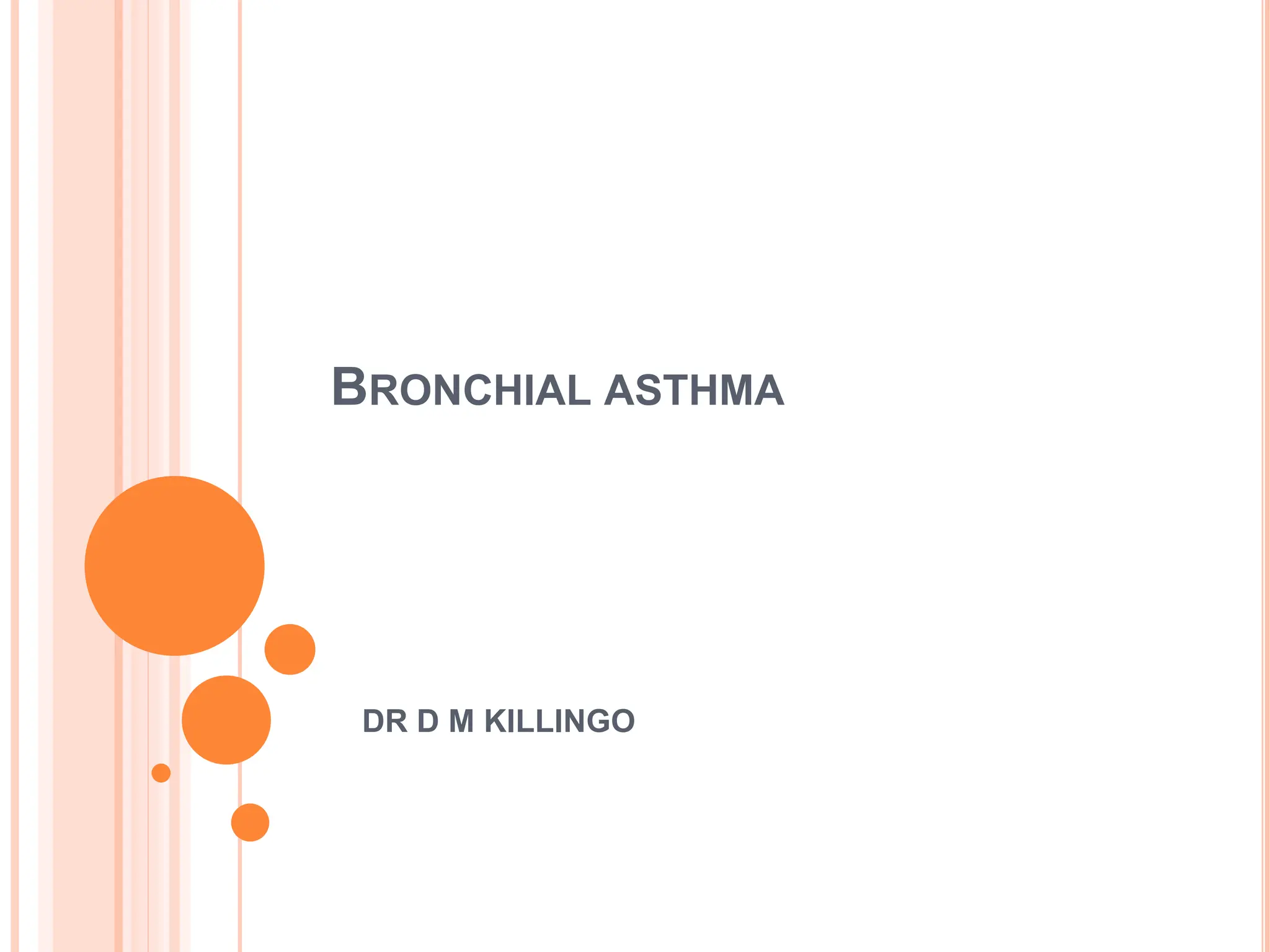
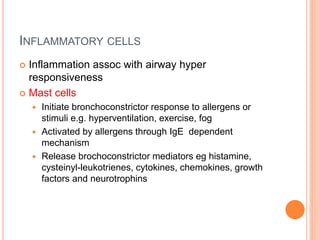
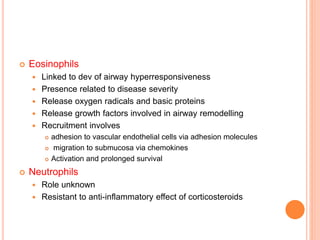
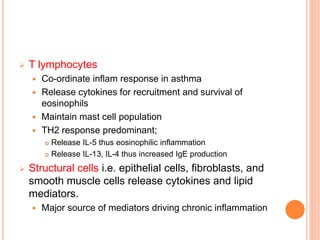
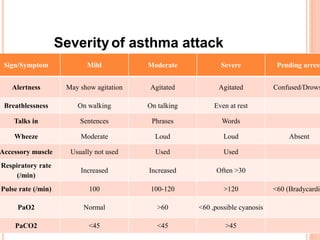
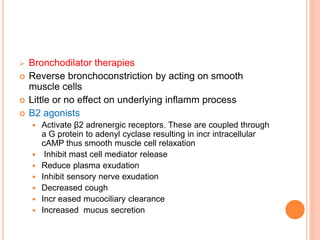
- Pathologically, it involves eosinophilic inflammation and thickening of the airway walls. Clinically, it presents with wheezing, coughing, and shortness of breath.
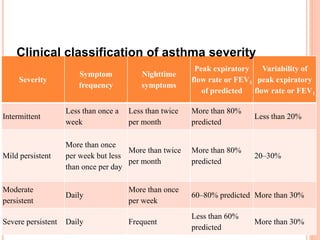
- Diagnosis involves lung function tests showing reversibility and