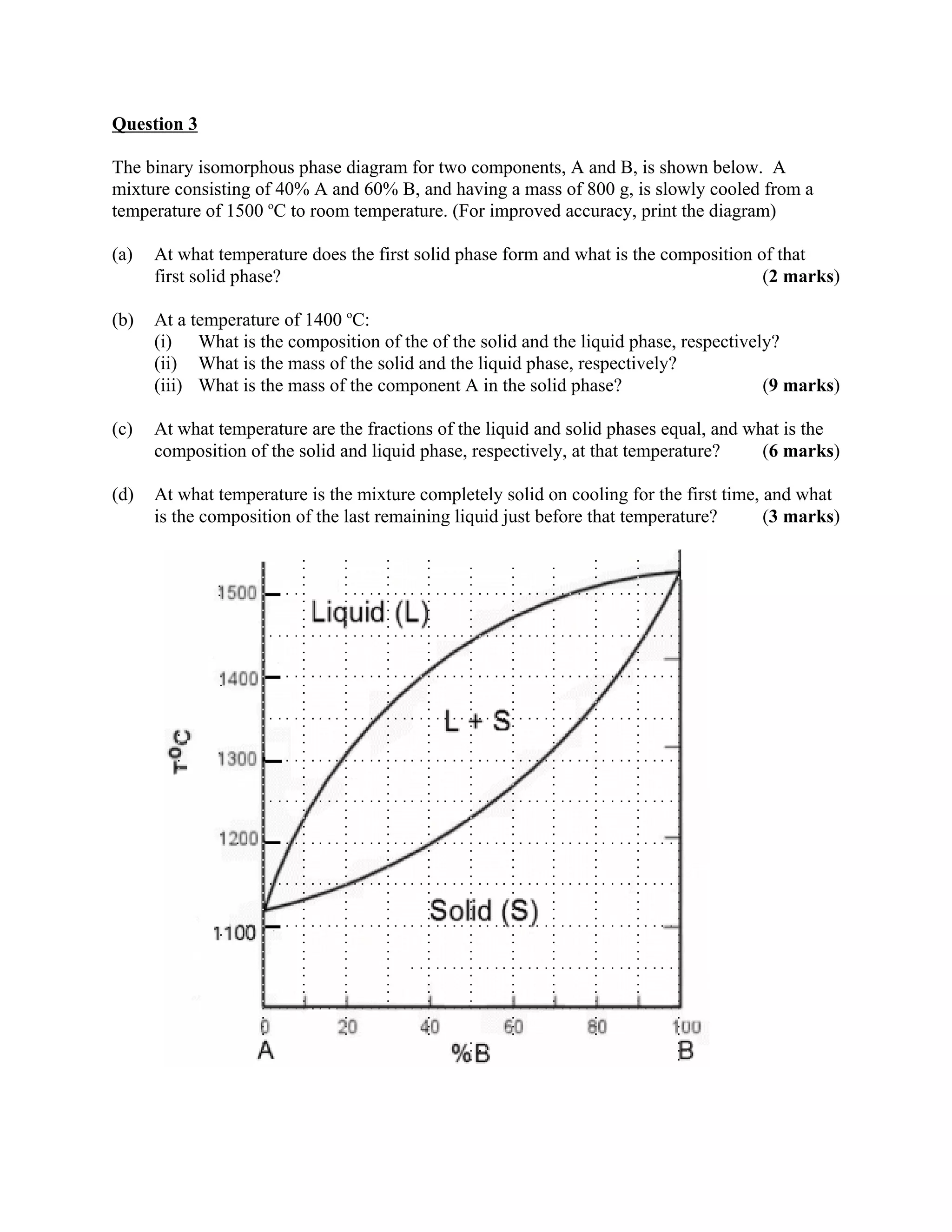
1. The document provides instructions for answering questions on a material science assignment involving phase diagrams and microstructures. Students are directed to show their work, include relevant details in their responses, and provide answers to two decimal places unless otherwise specified.
2. The assignment contains 4 questions involving distinguishing between octahedral and tetrahedral voids in FCC structures, explaining differences in carbon solubility between austenite and ferrite, using a phase diagram to analyze an Fe-C alloy, and analyzing cooling processes using a binary phase diagram.