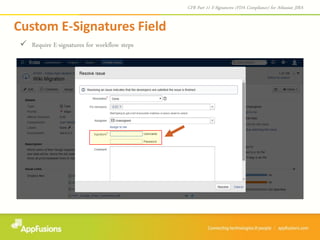
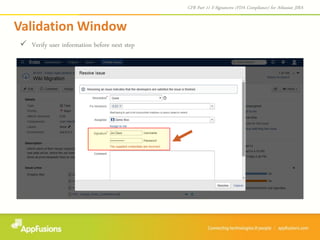
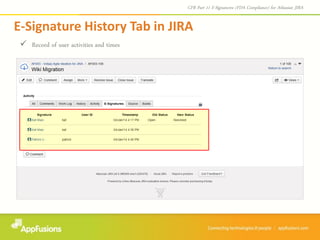
The document describes a JIRA add-on that provides e-signature and audit reporting capabilities to comply with FDA CFR Part 11 regulations. The add-on adds an e-signature field to the JIRA workflow where users must authenticate before transitioning an issue to the next stage. It also provides an audit report with user names, dates, and timestamps of e-signatures that can be printed from within JIRA. The add-on comes with a free 30-day trial and is designed to integrate seamlessly with JIRA.