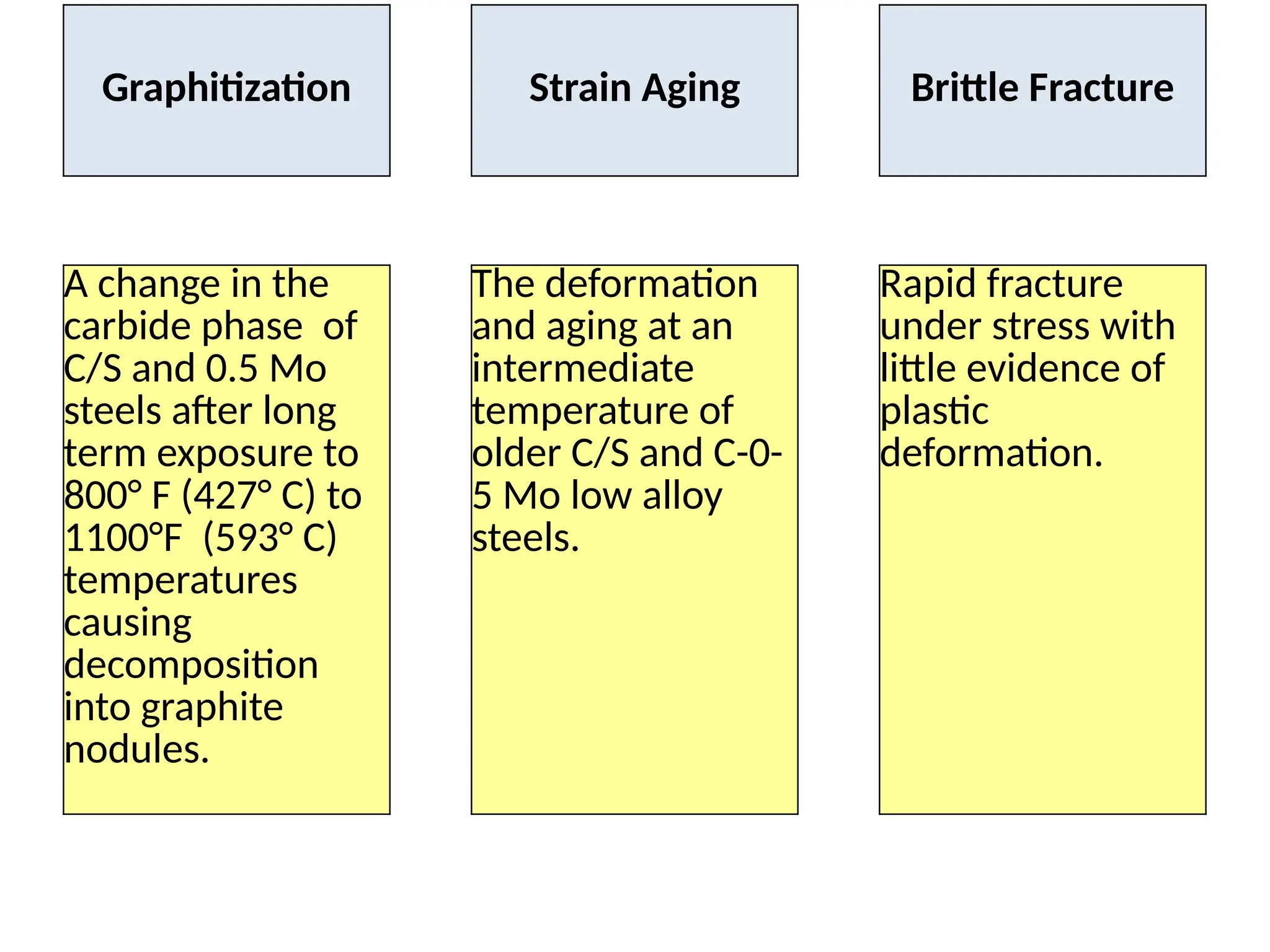
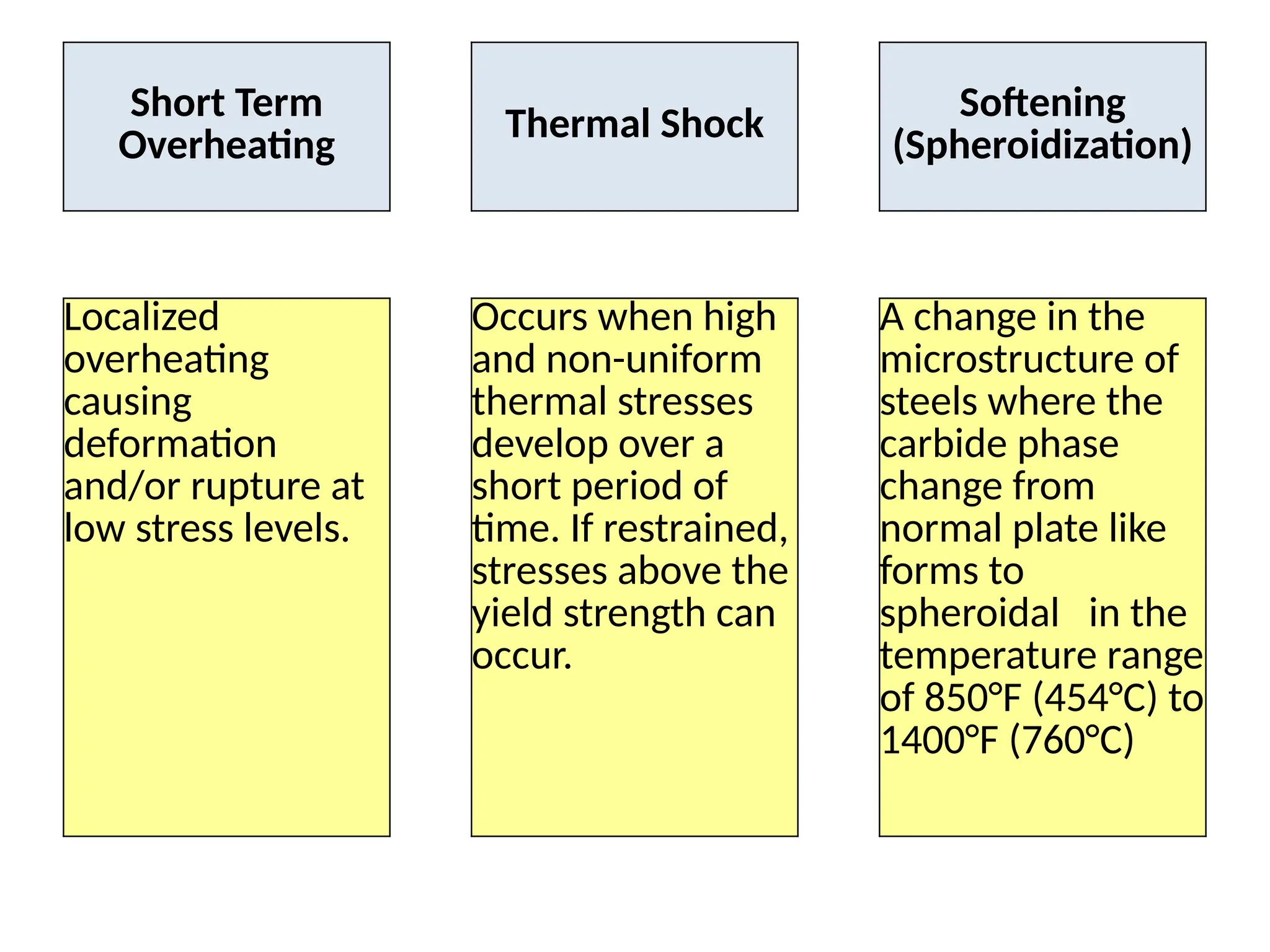
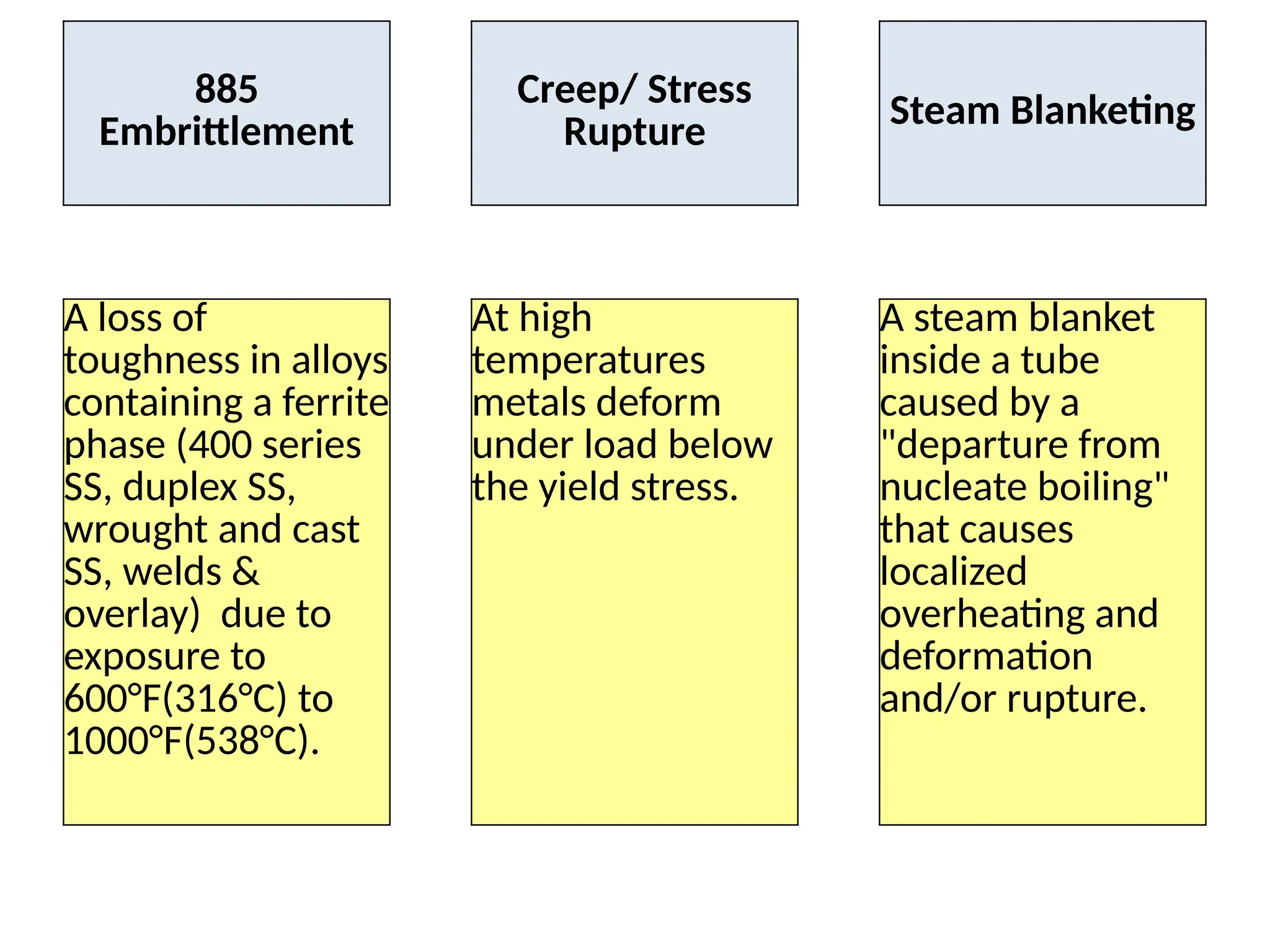
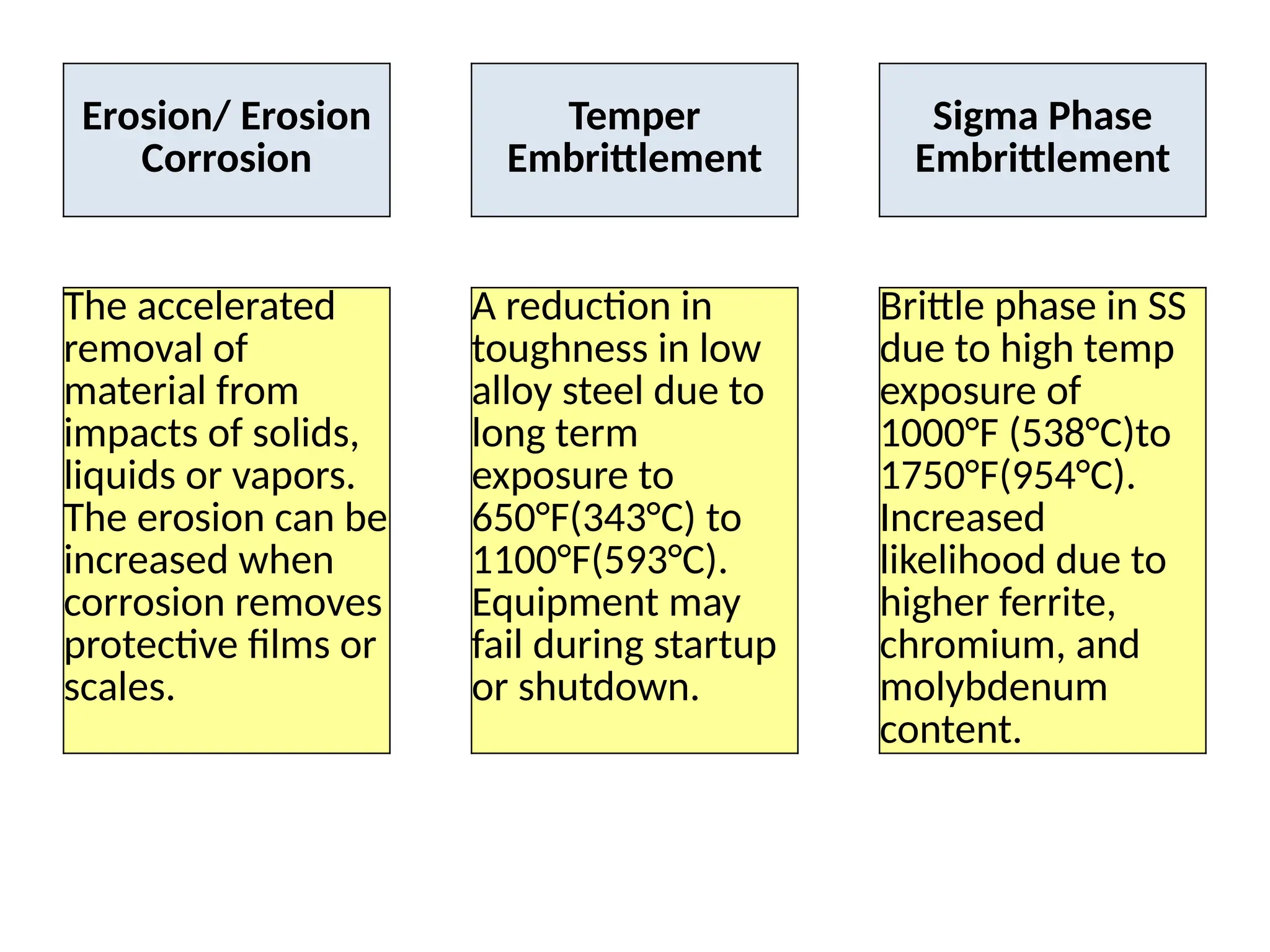
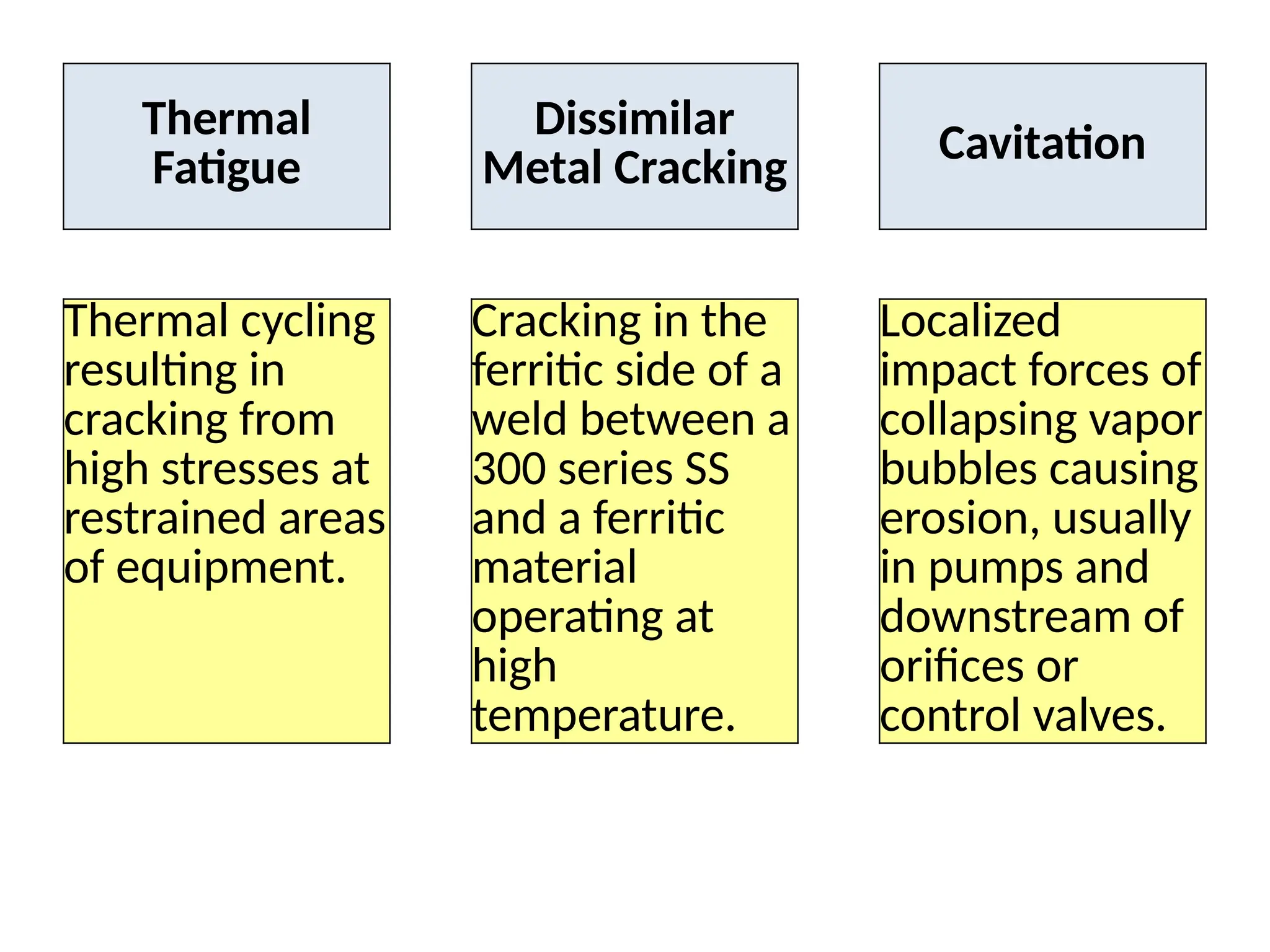
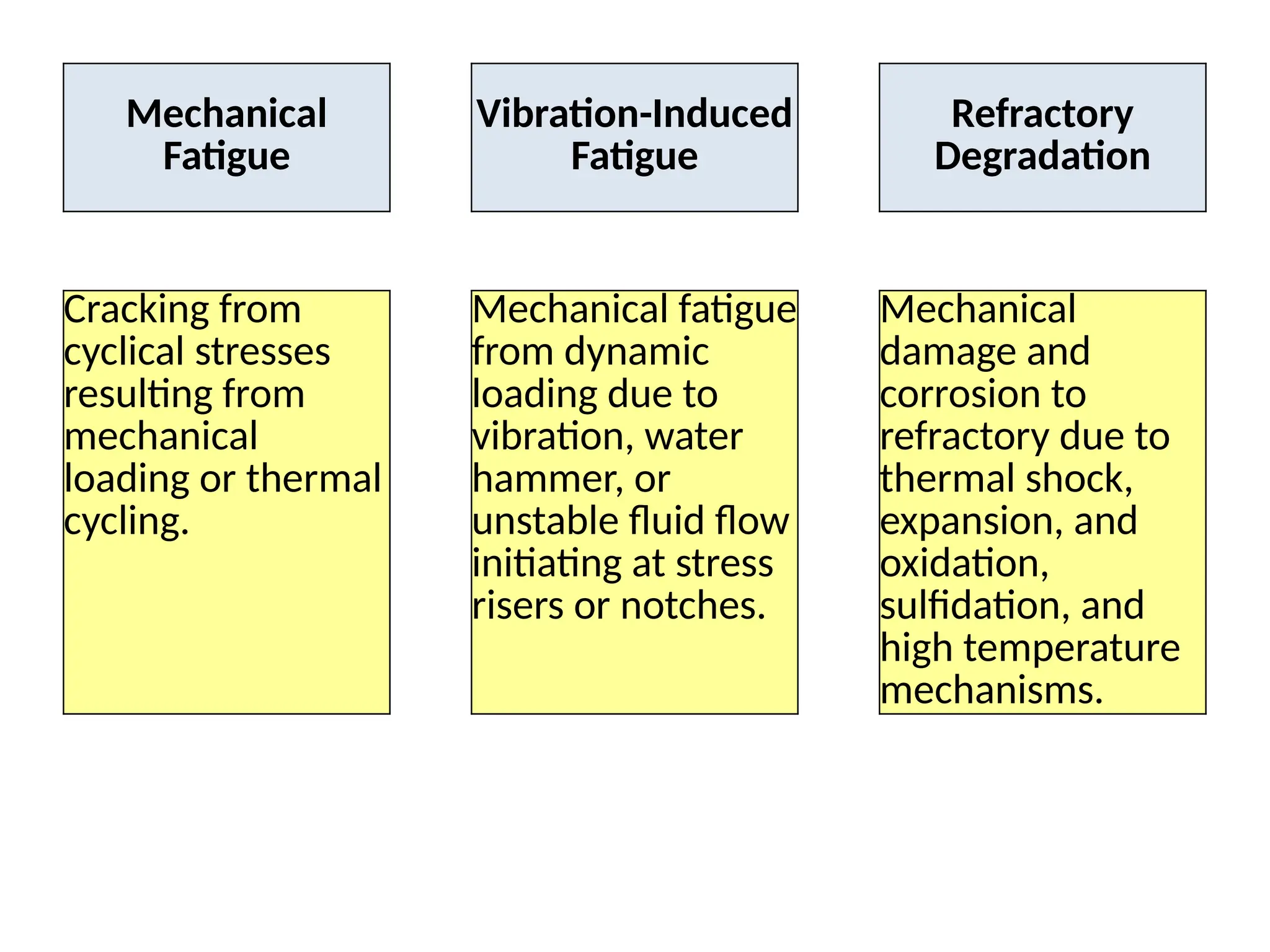
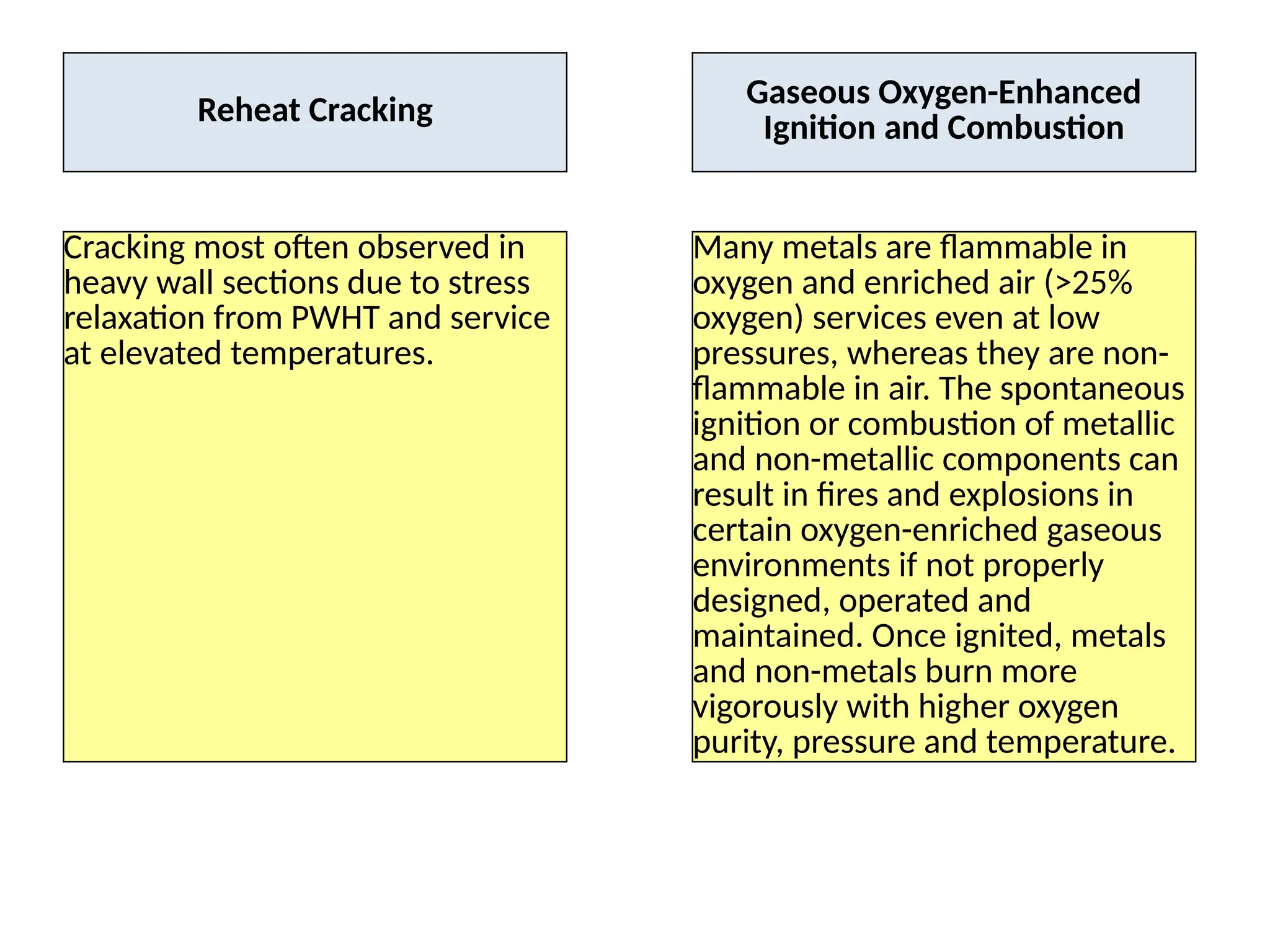
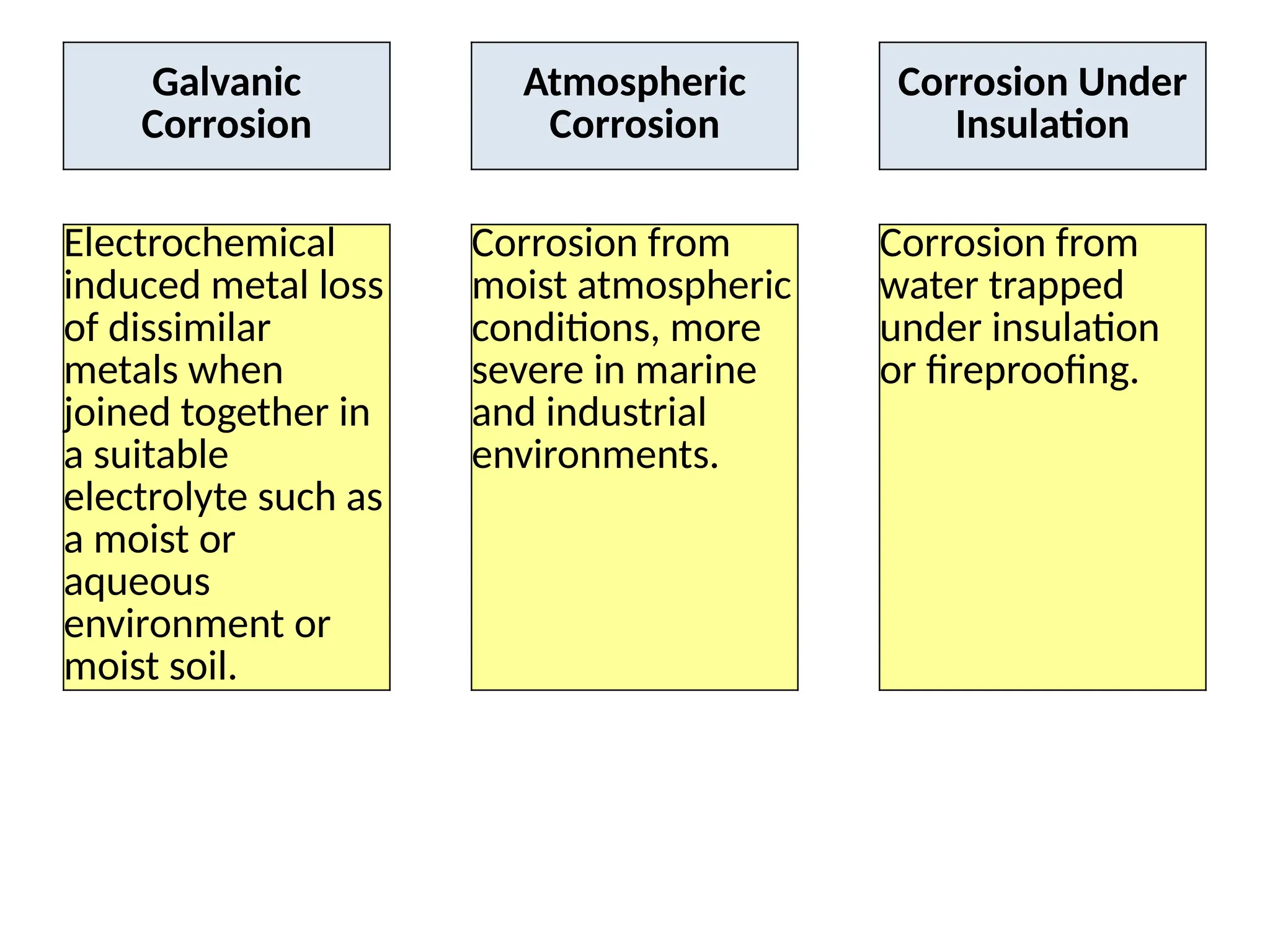
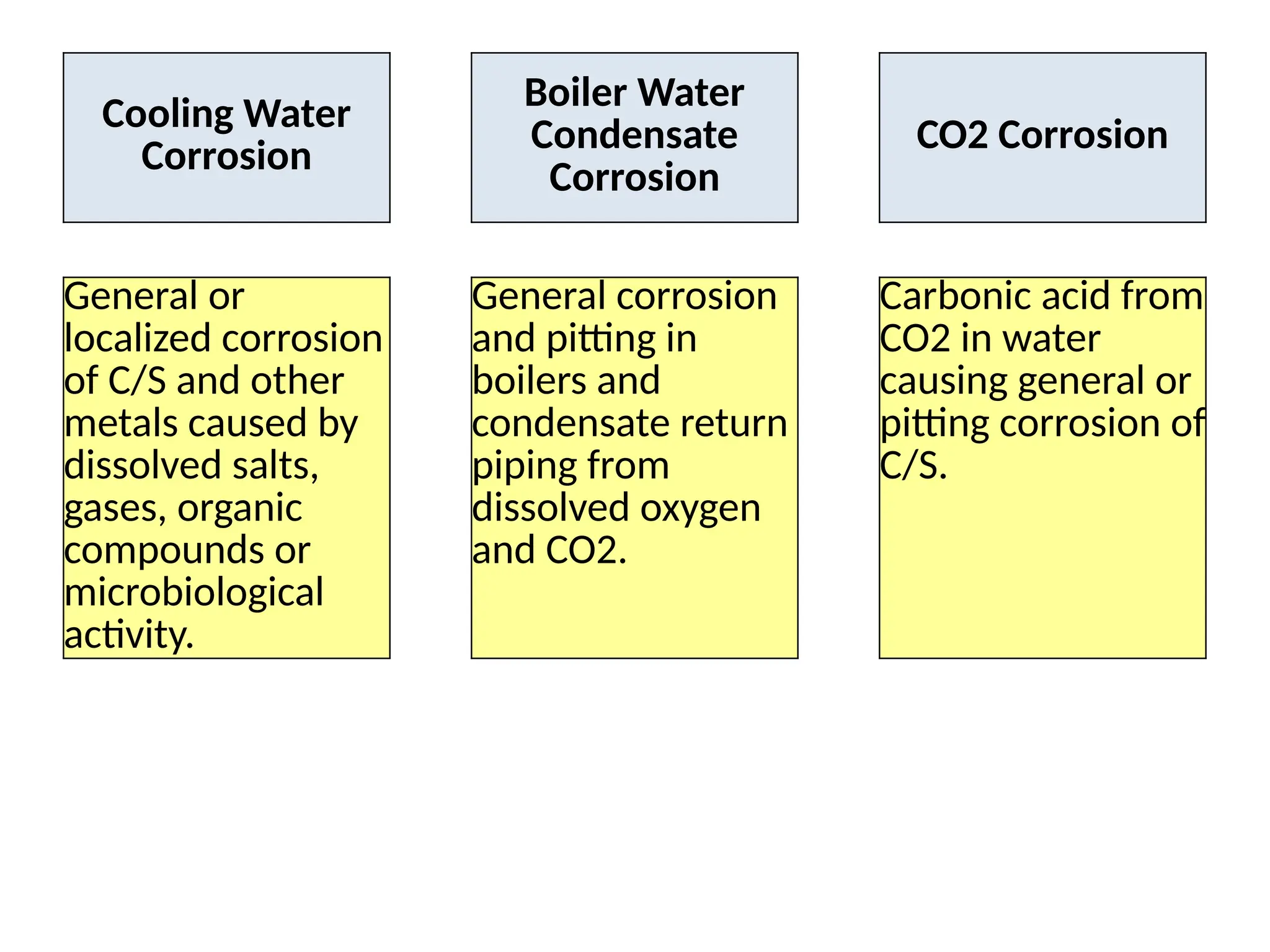
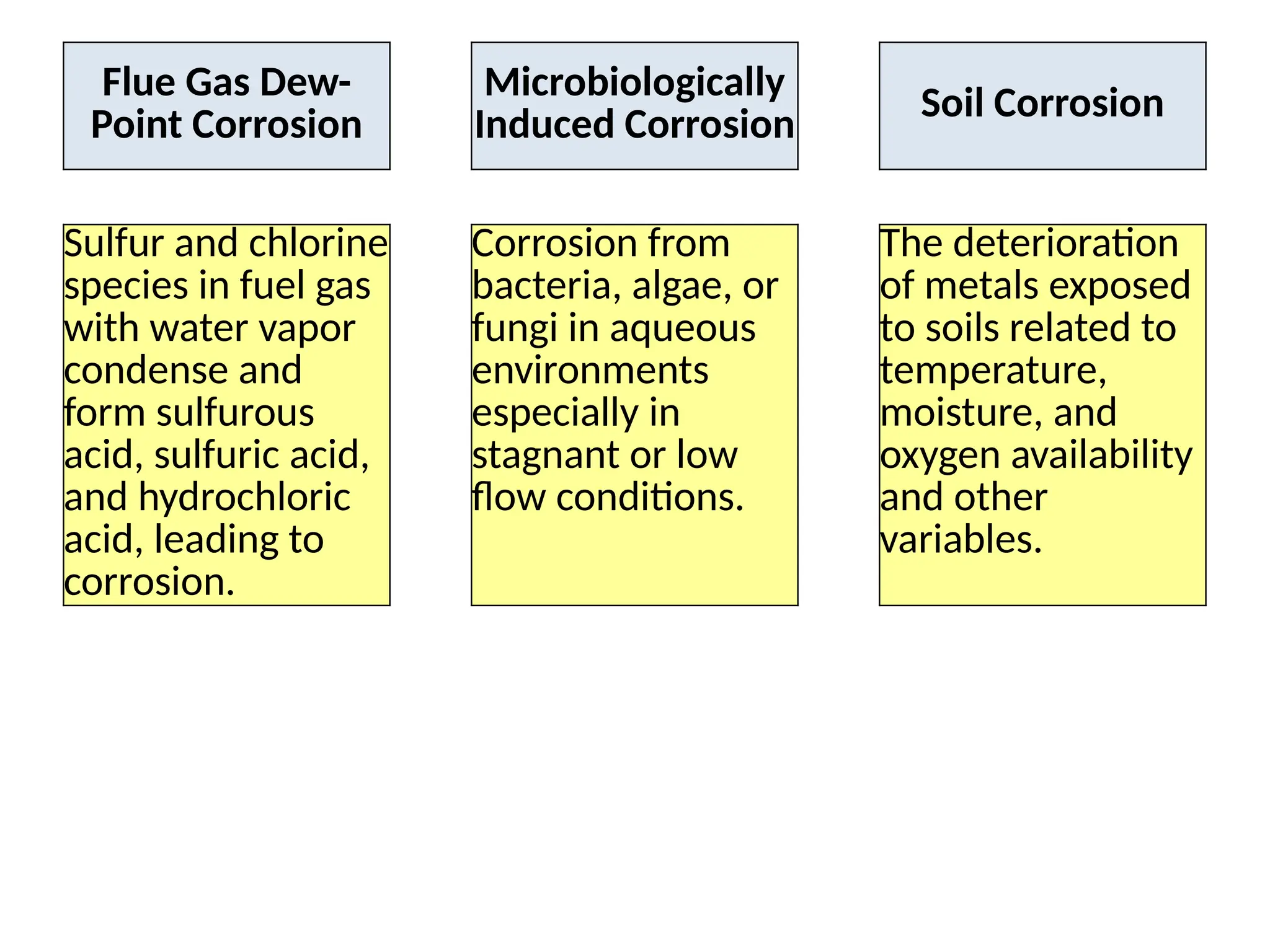
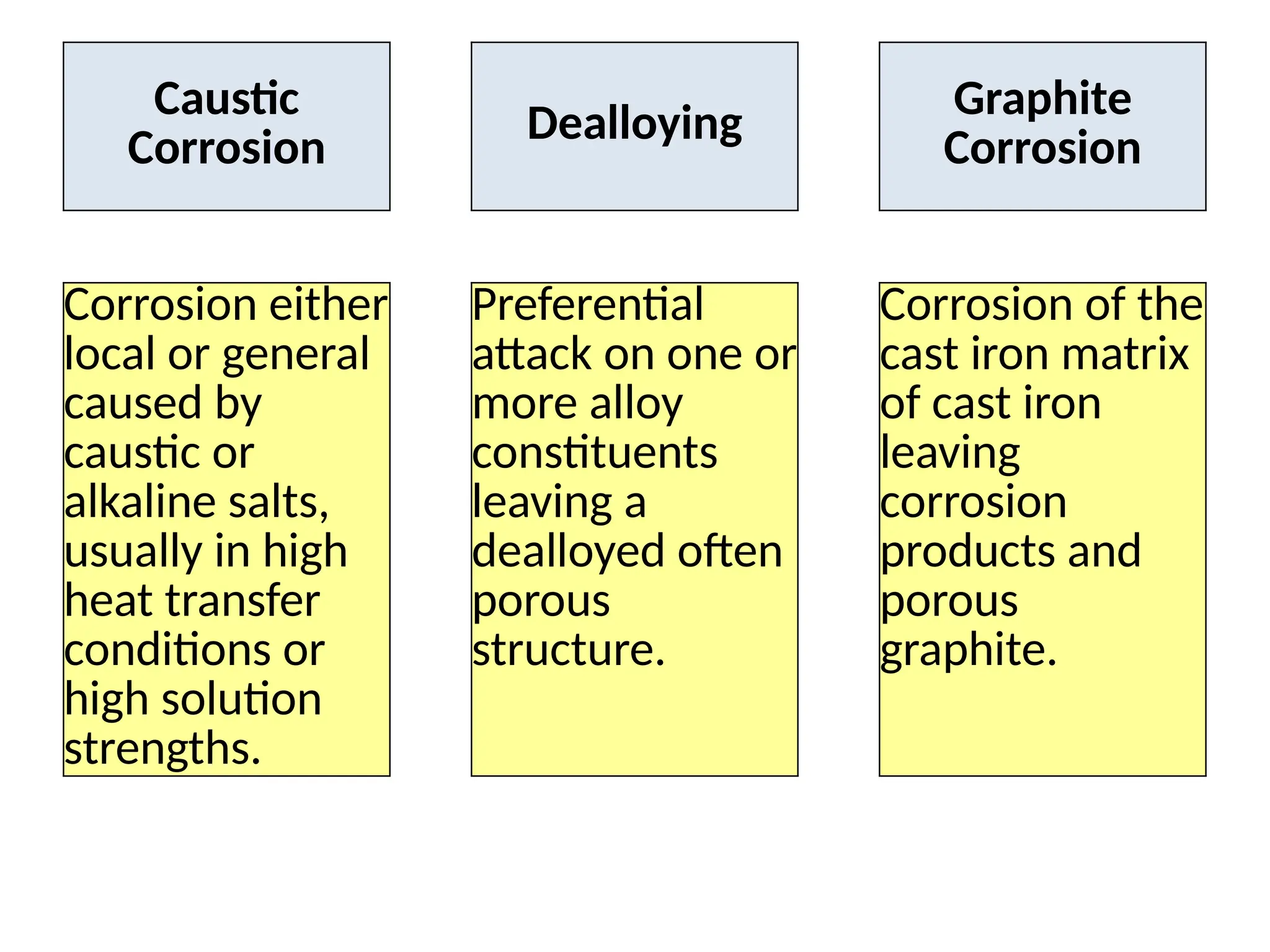
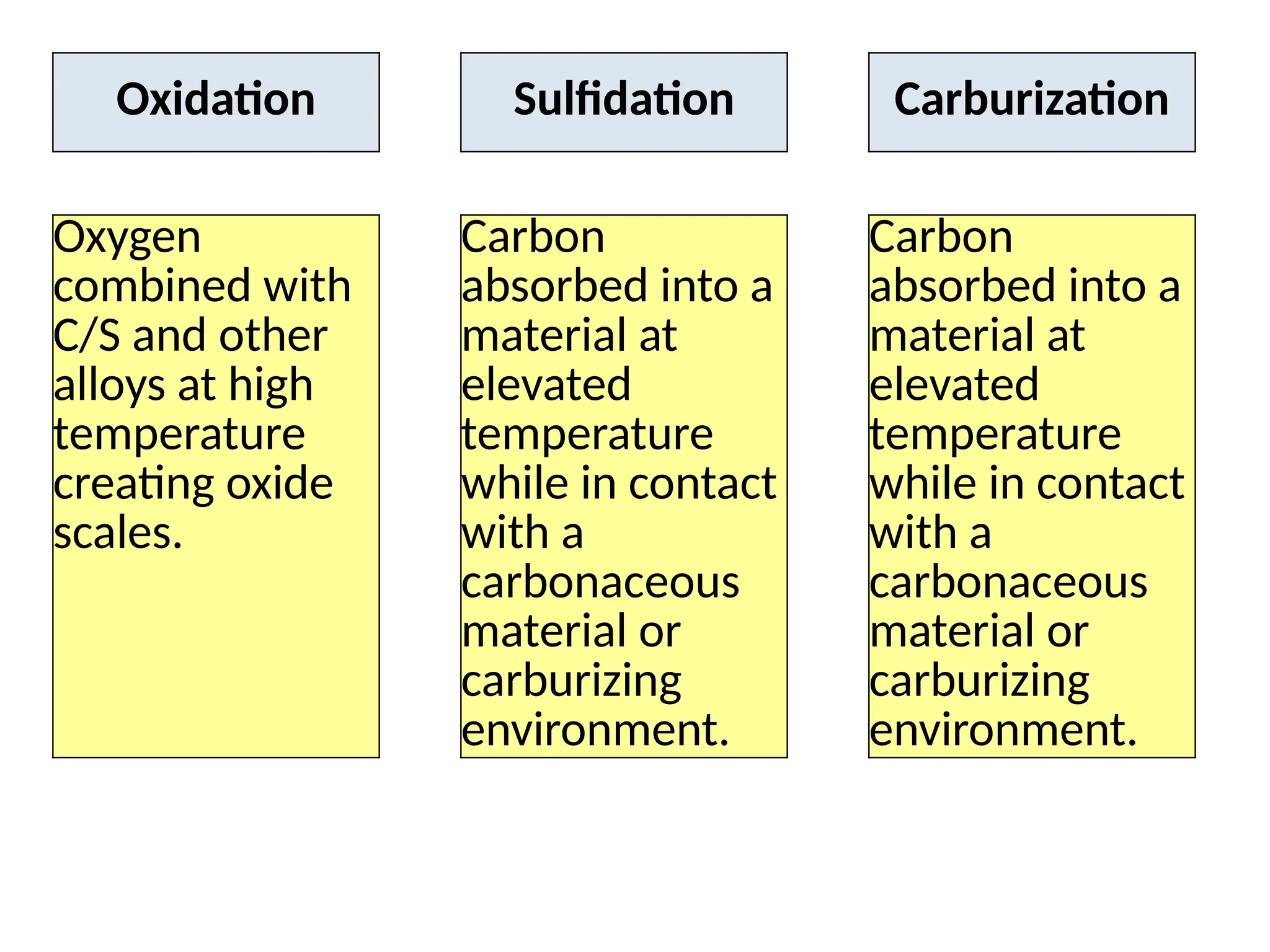
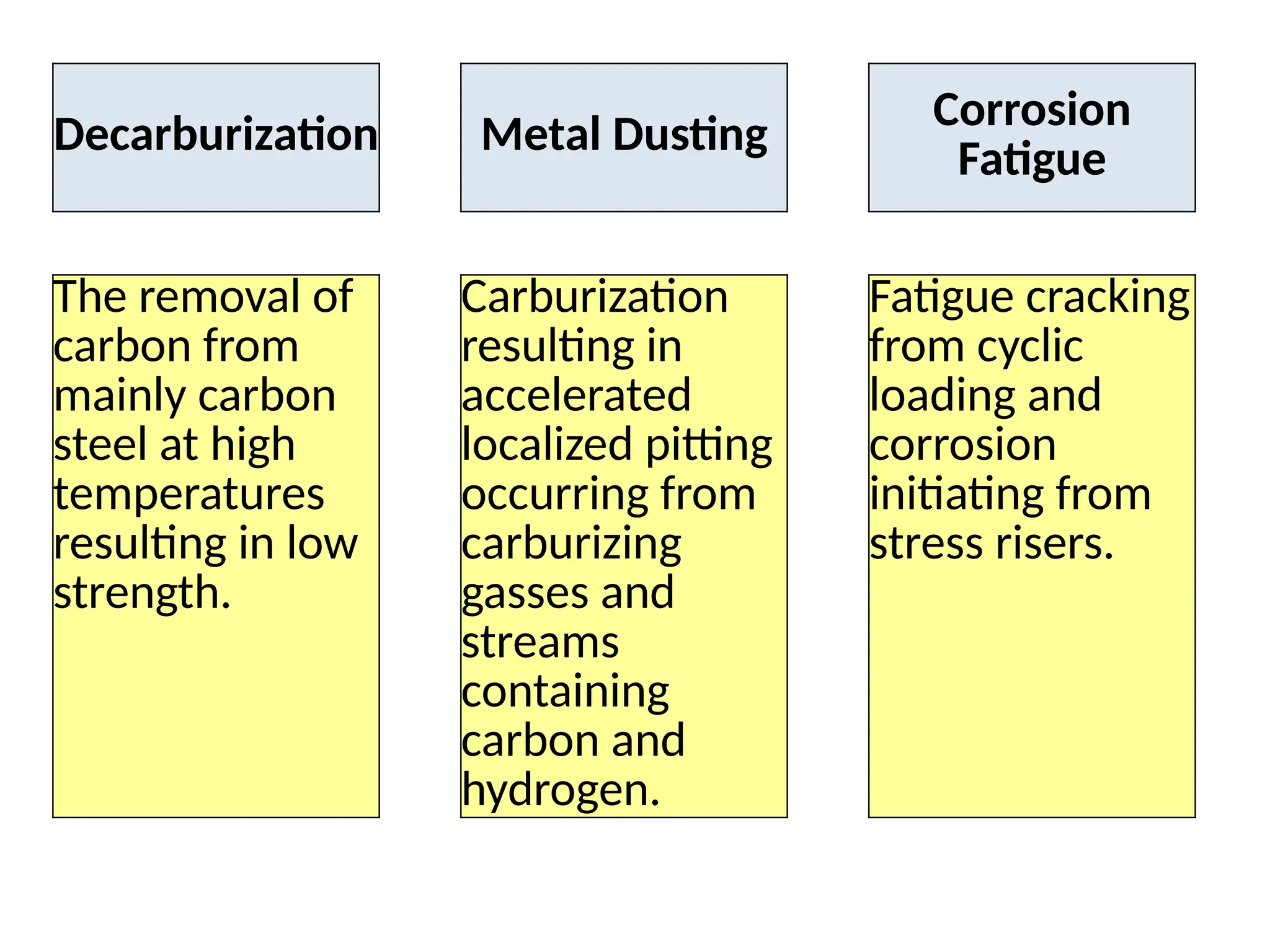
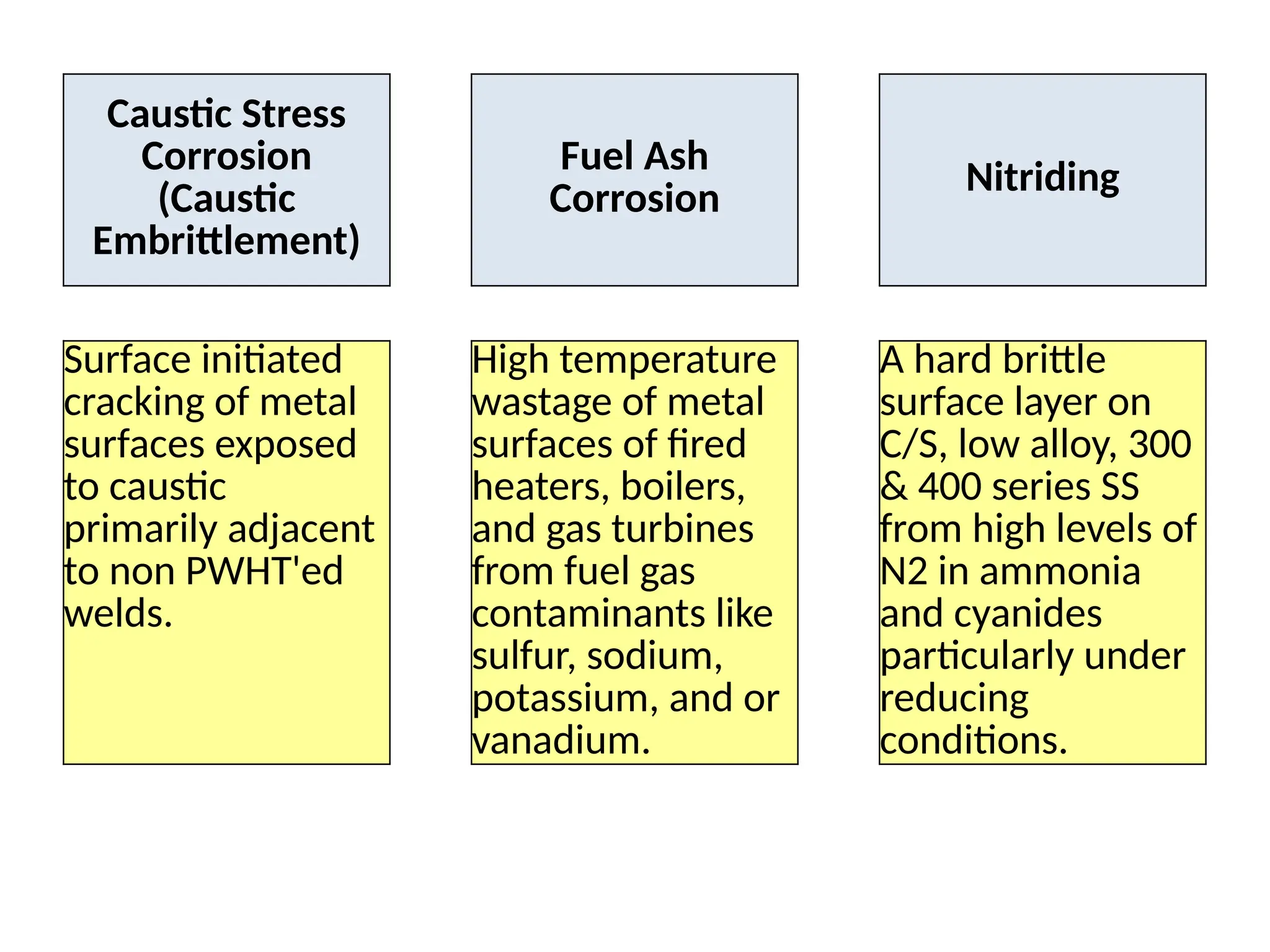
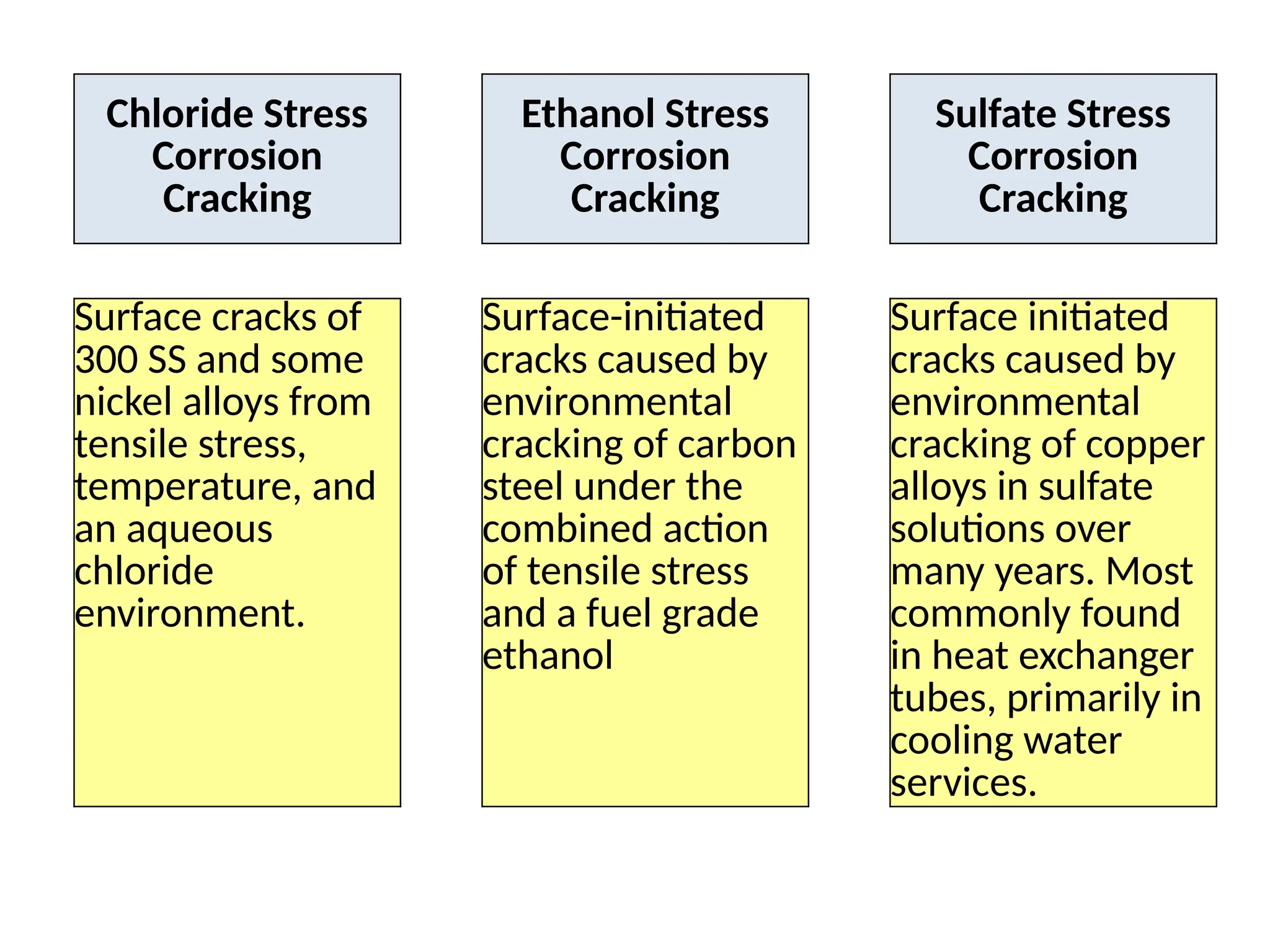
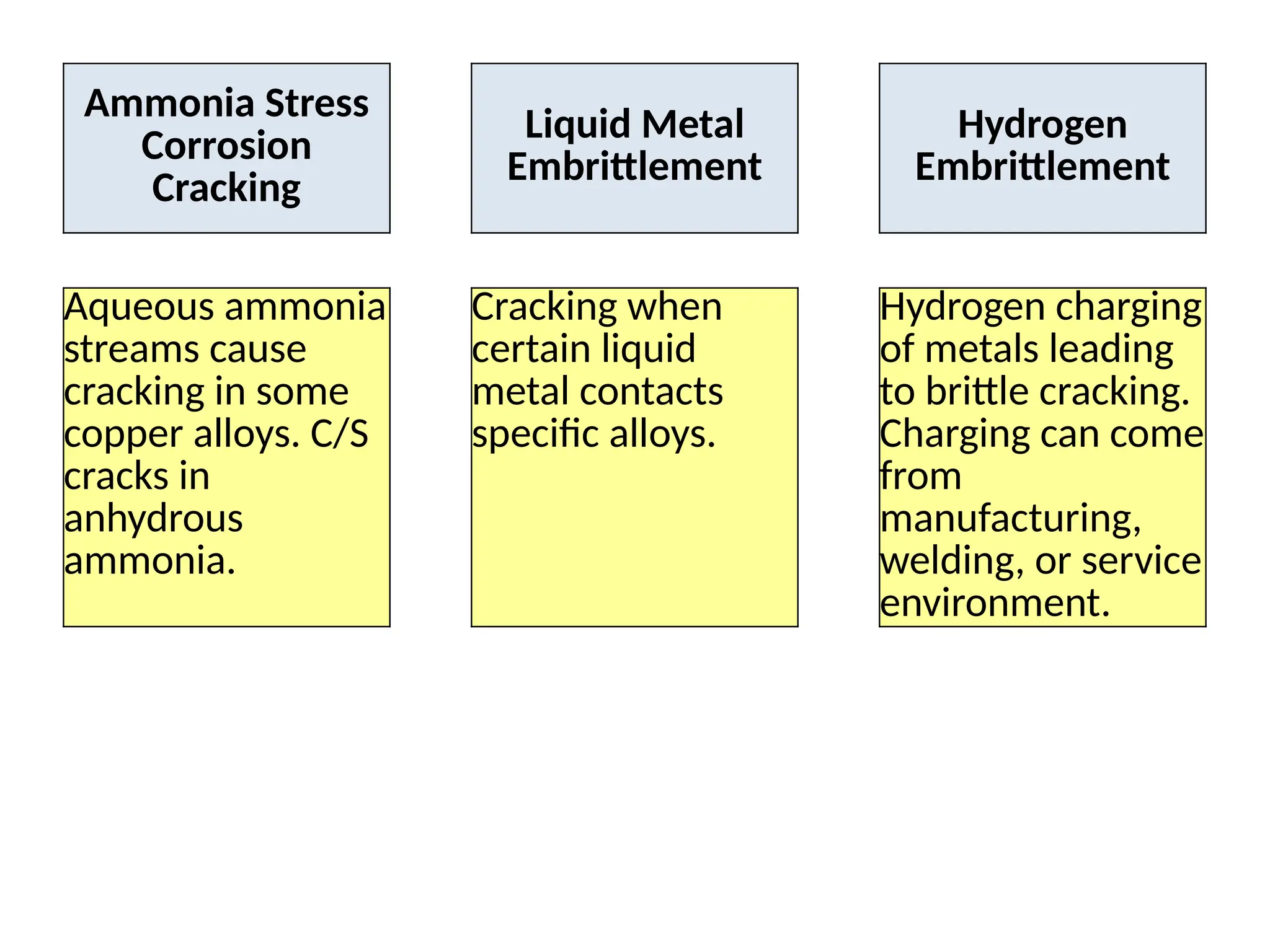
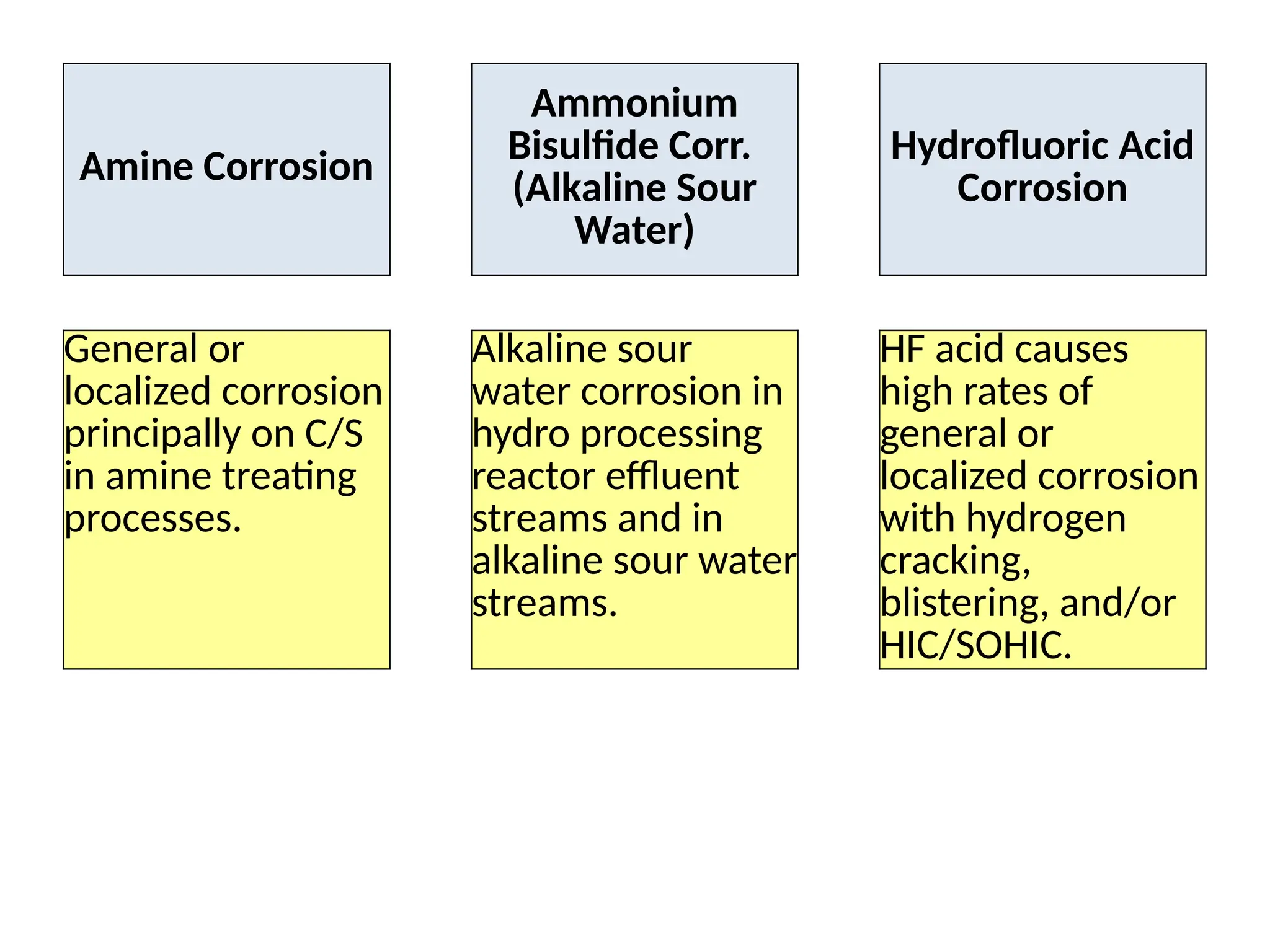
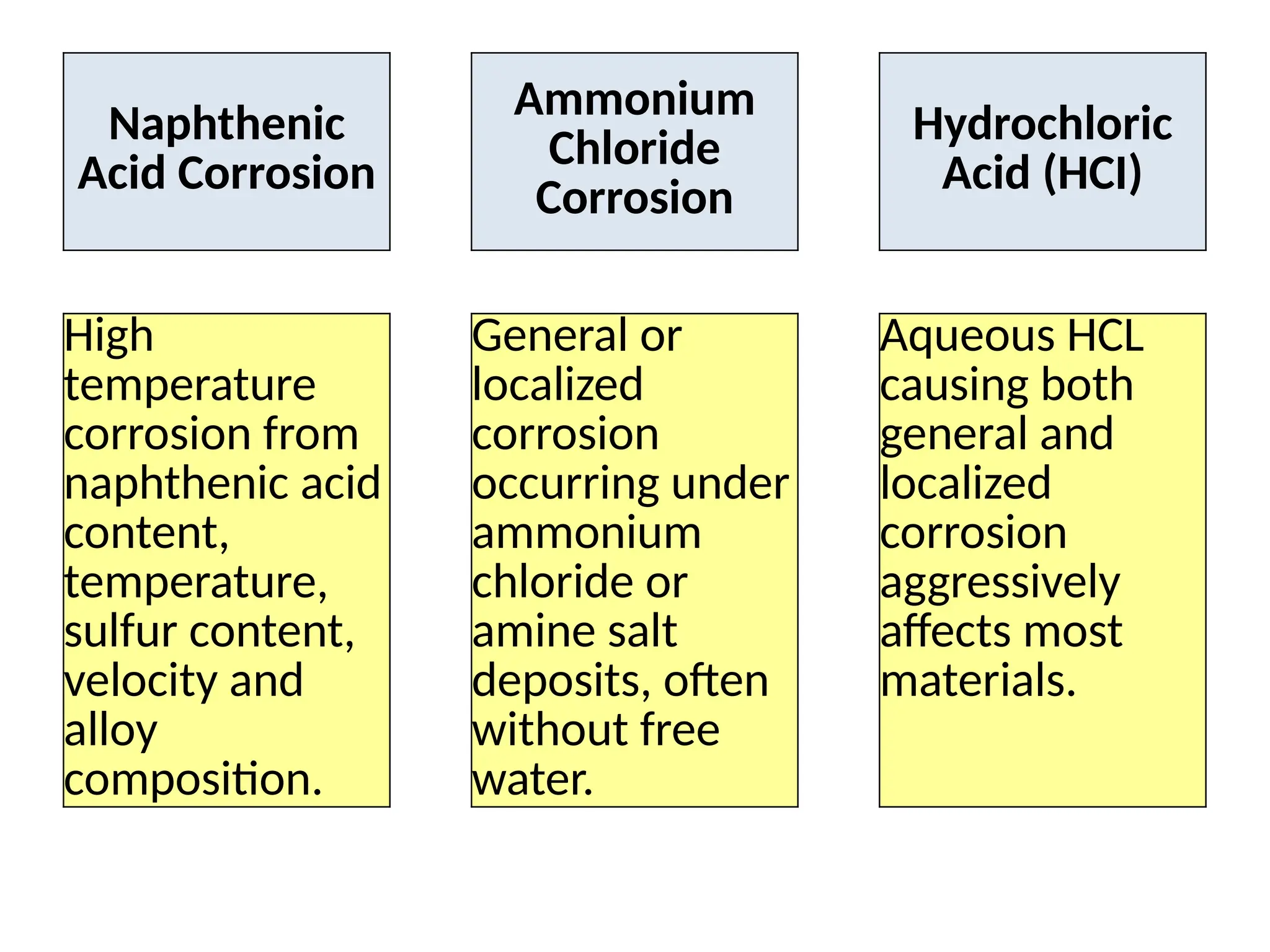
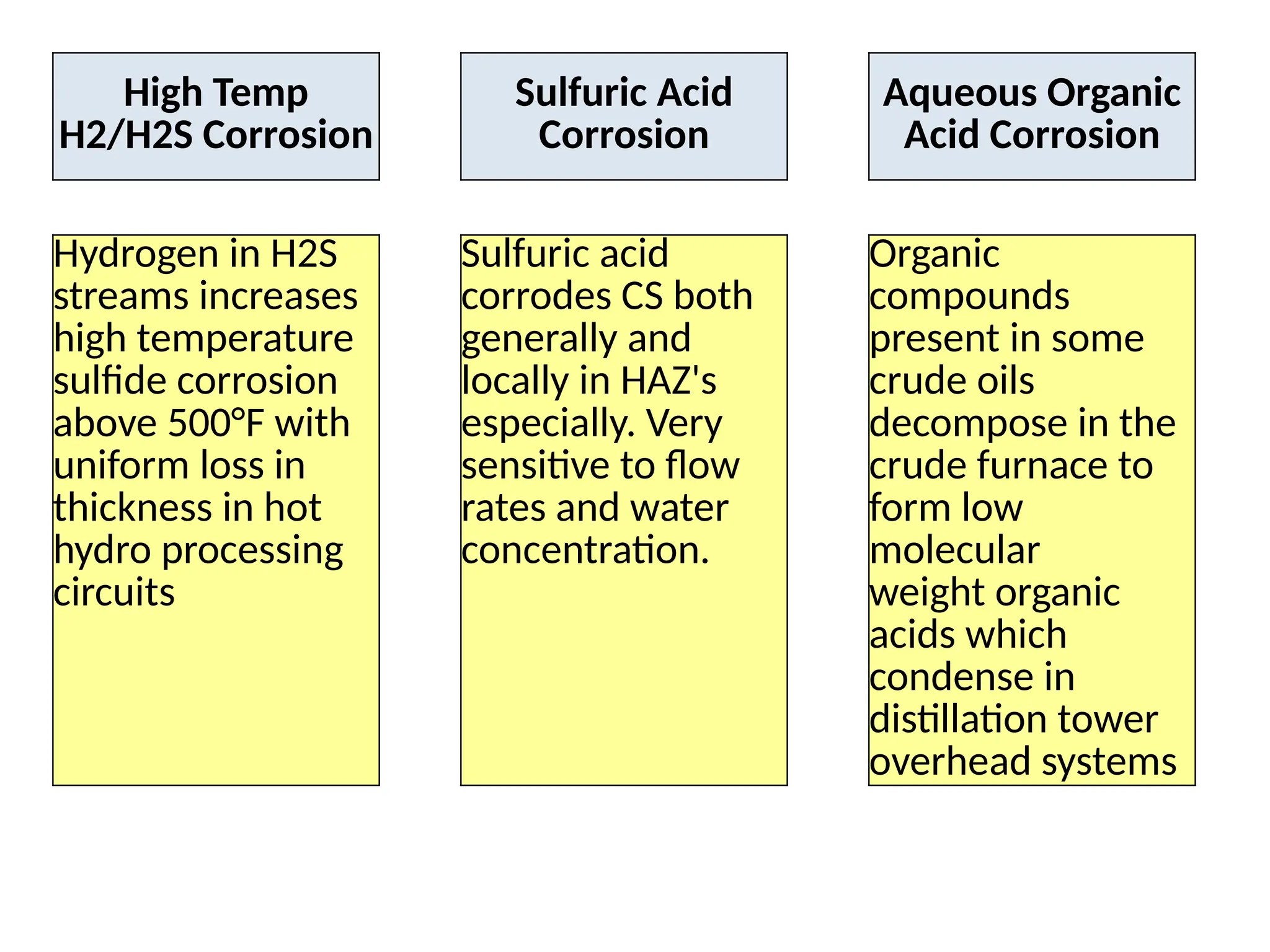
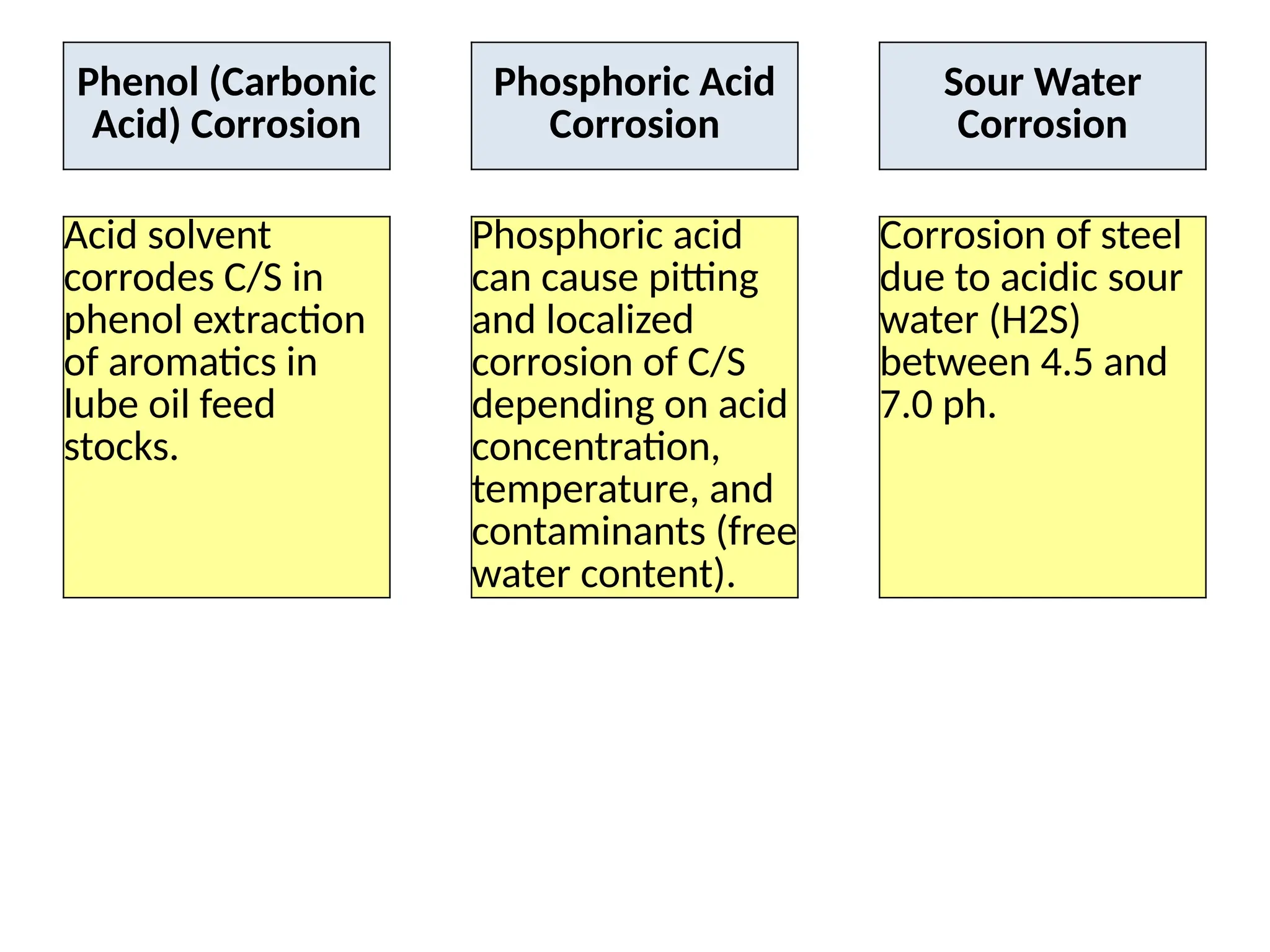
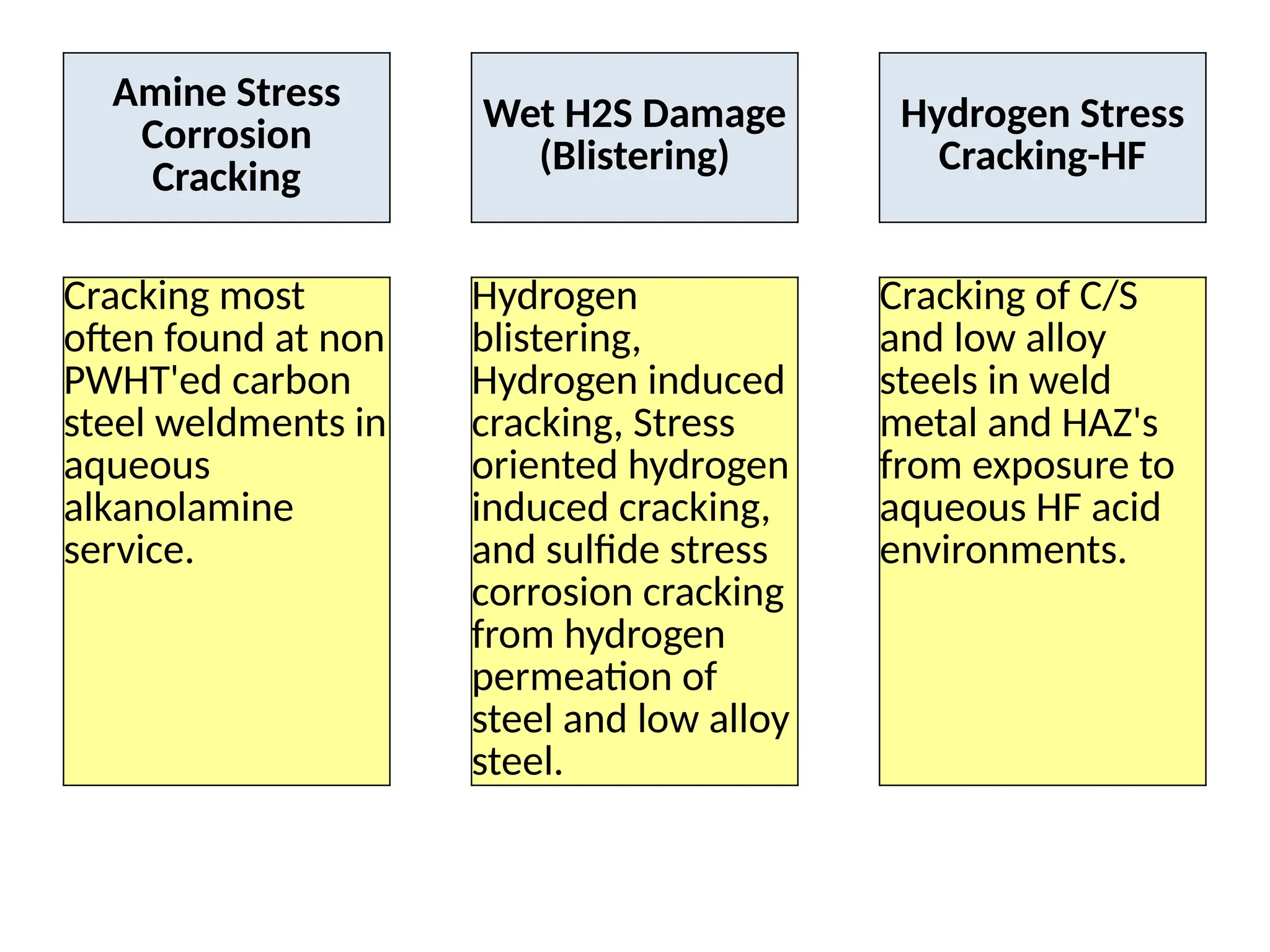
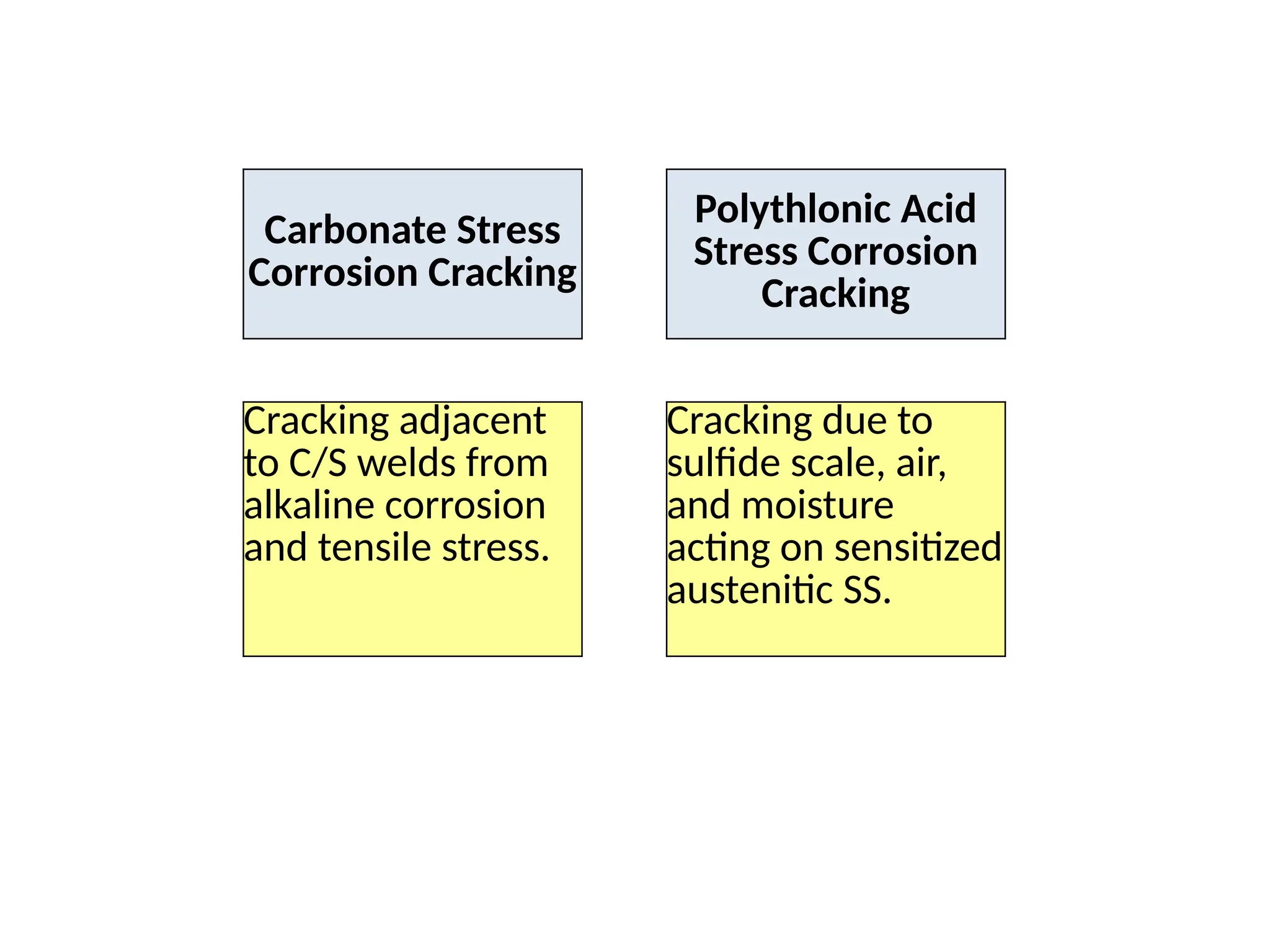
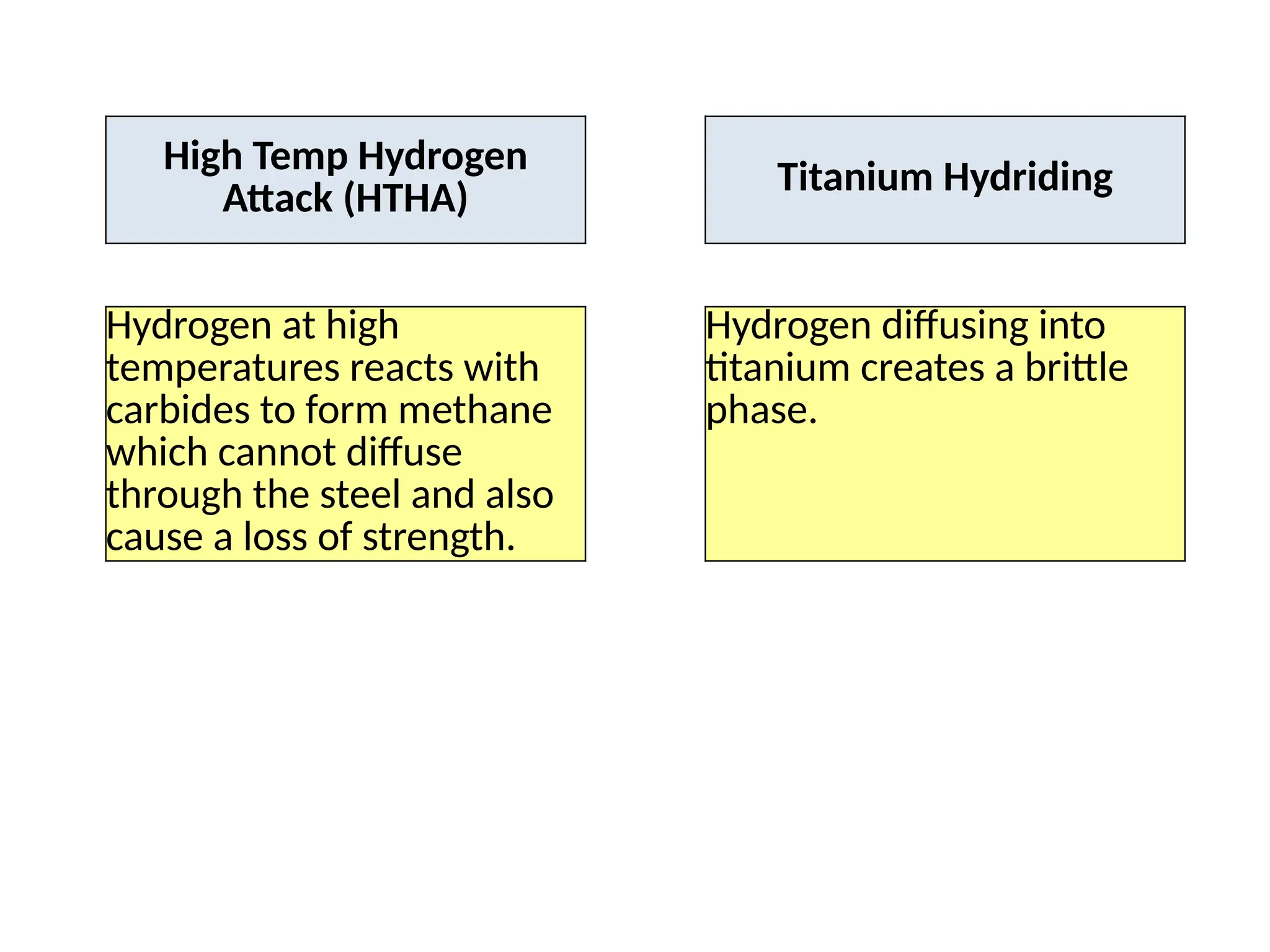
The document outlines various damage mechanisms that affect mechanical and metallurgical components, highlighting phenomena such as graphitization, brittle fracture, and corrosion in different environments. It details temperature-related failures, the effects of stress, and specific corrosive agents leading to material degradation. The mechanisms discussed are critical for understanding and preventing equipment failures in industrial settings.