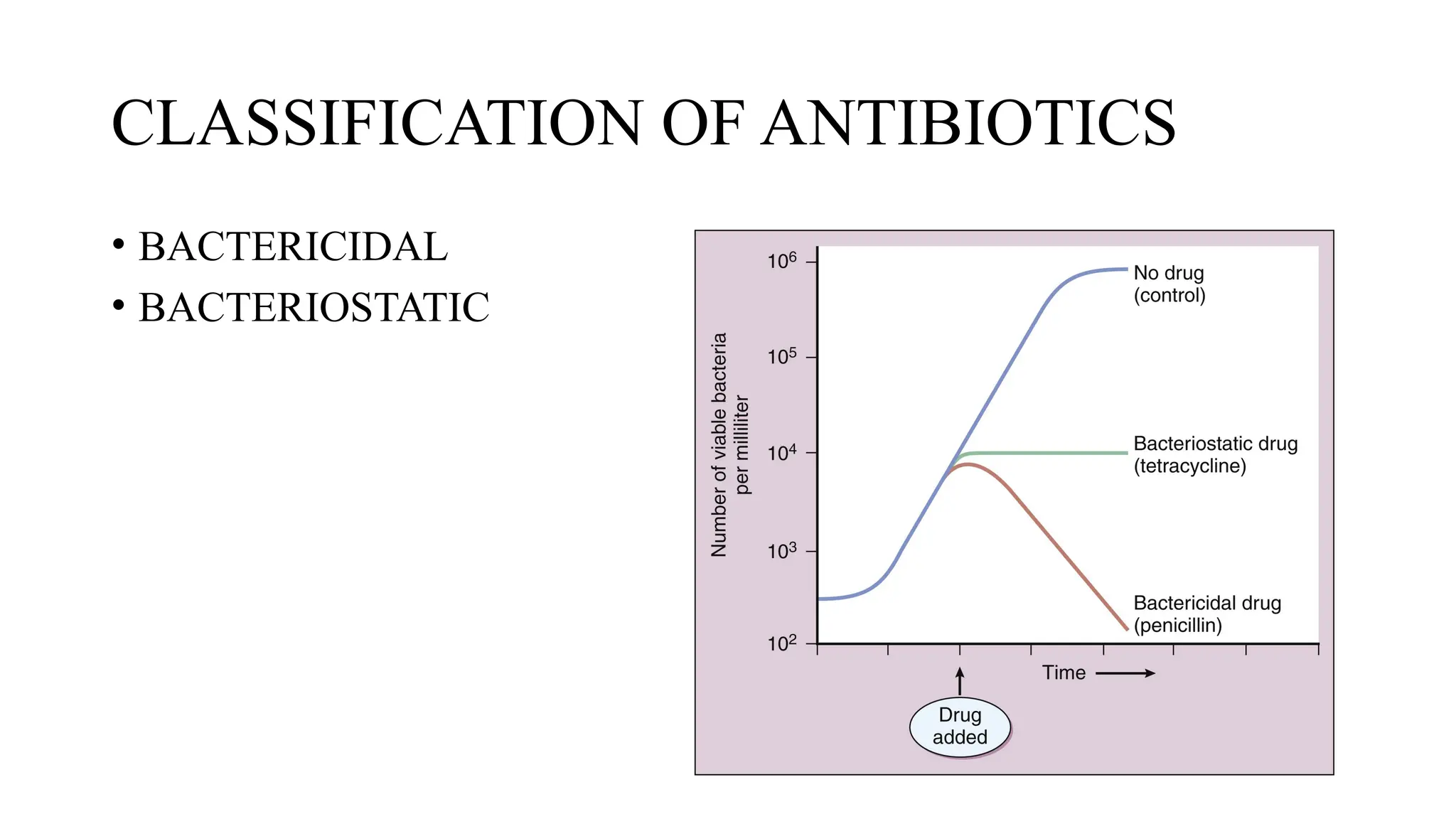
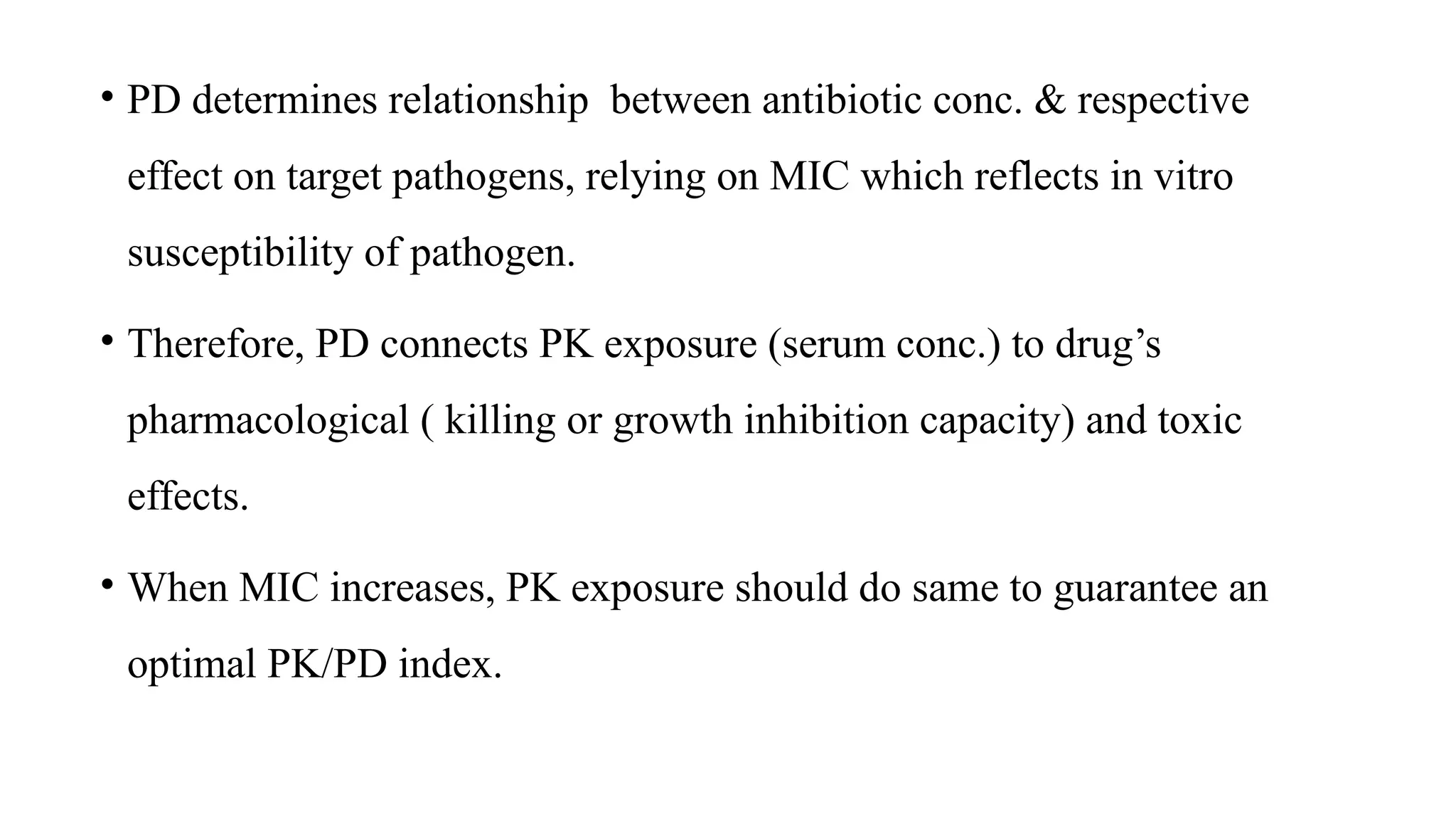
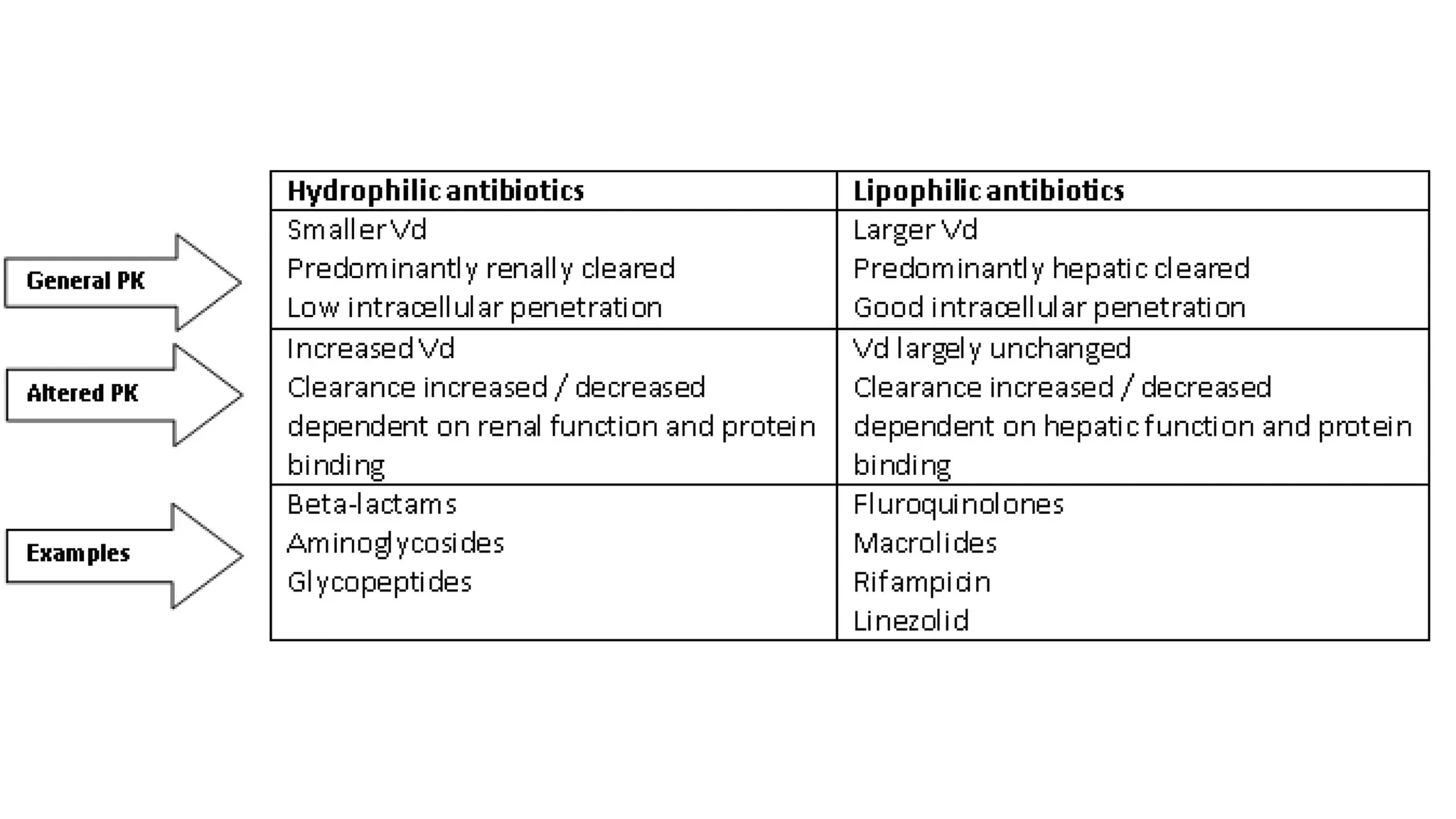
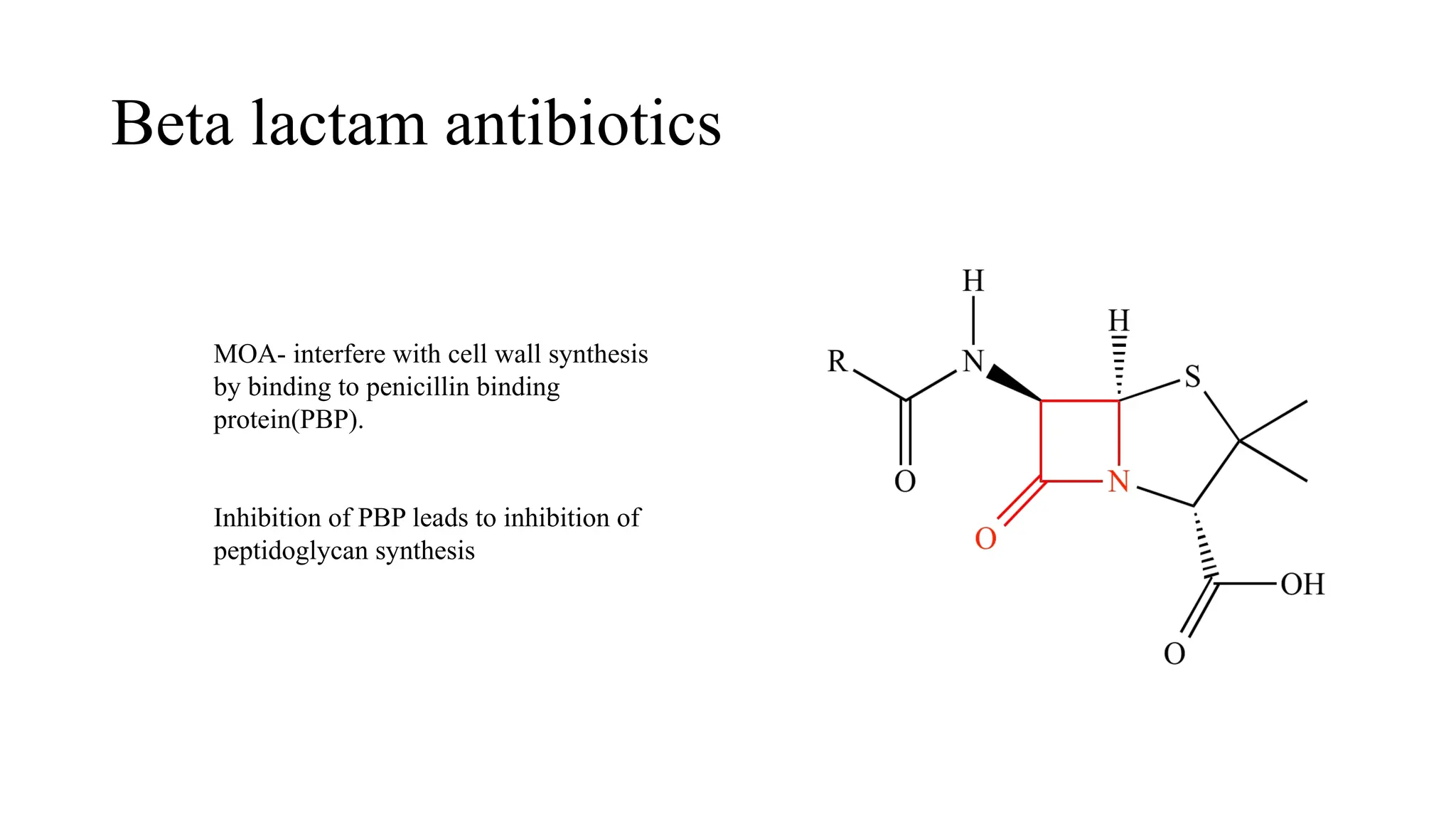
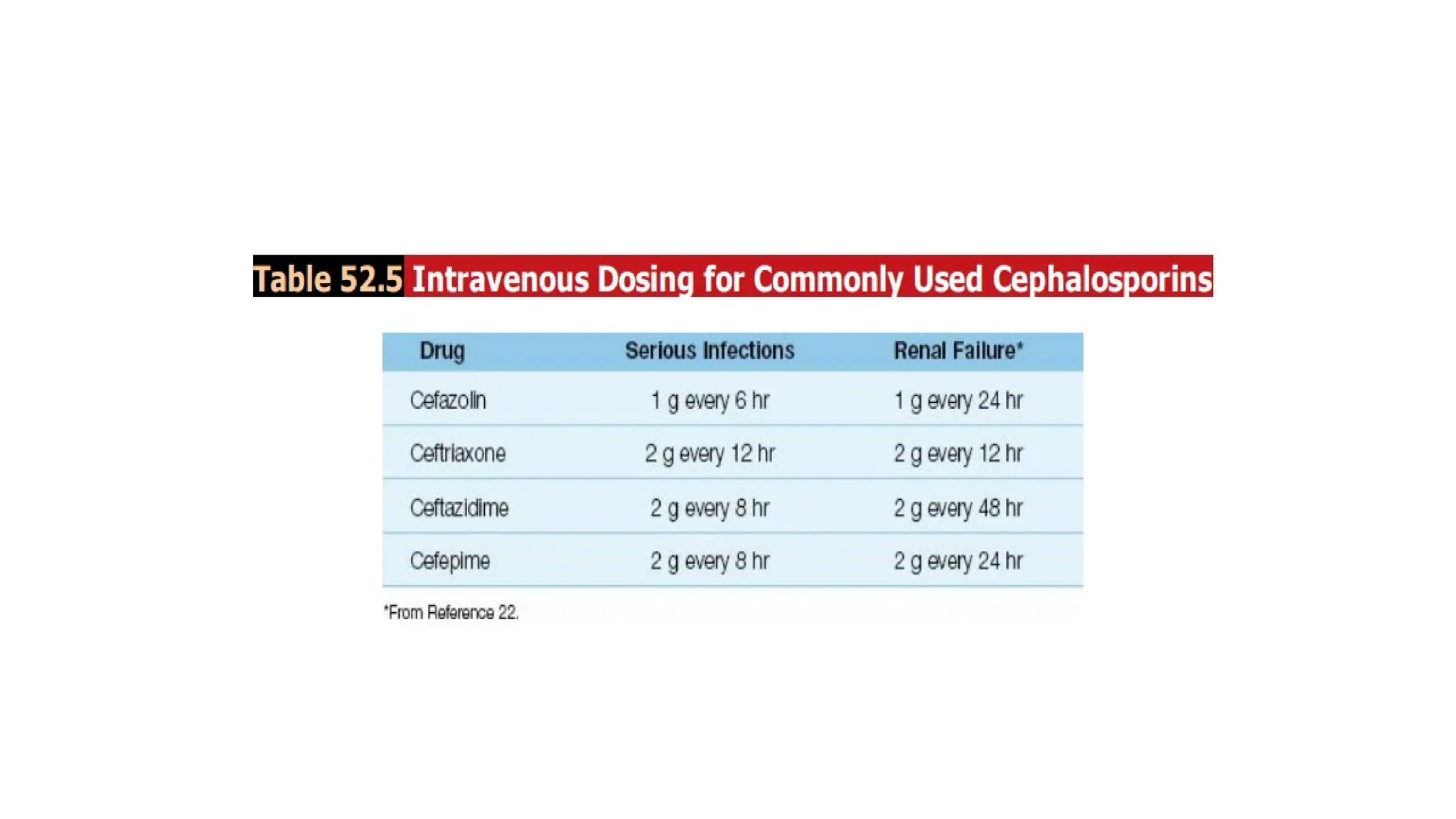
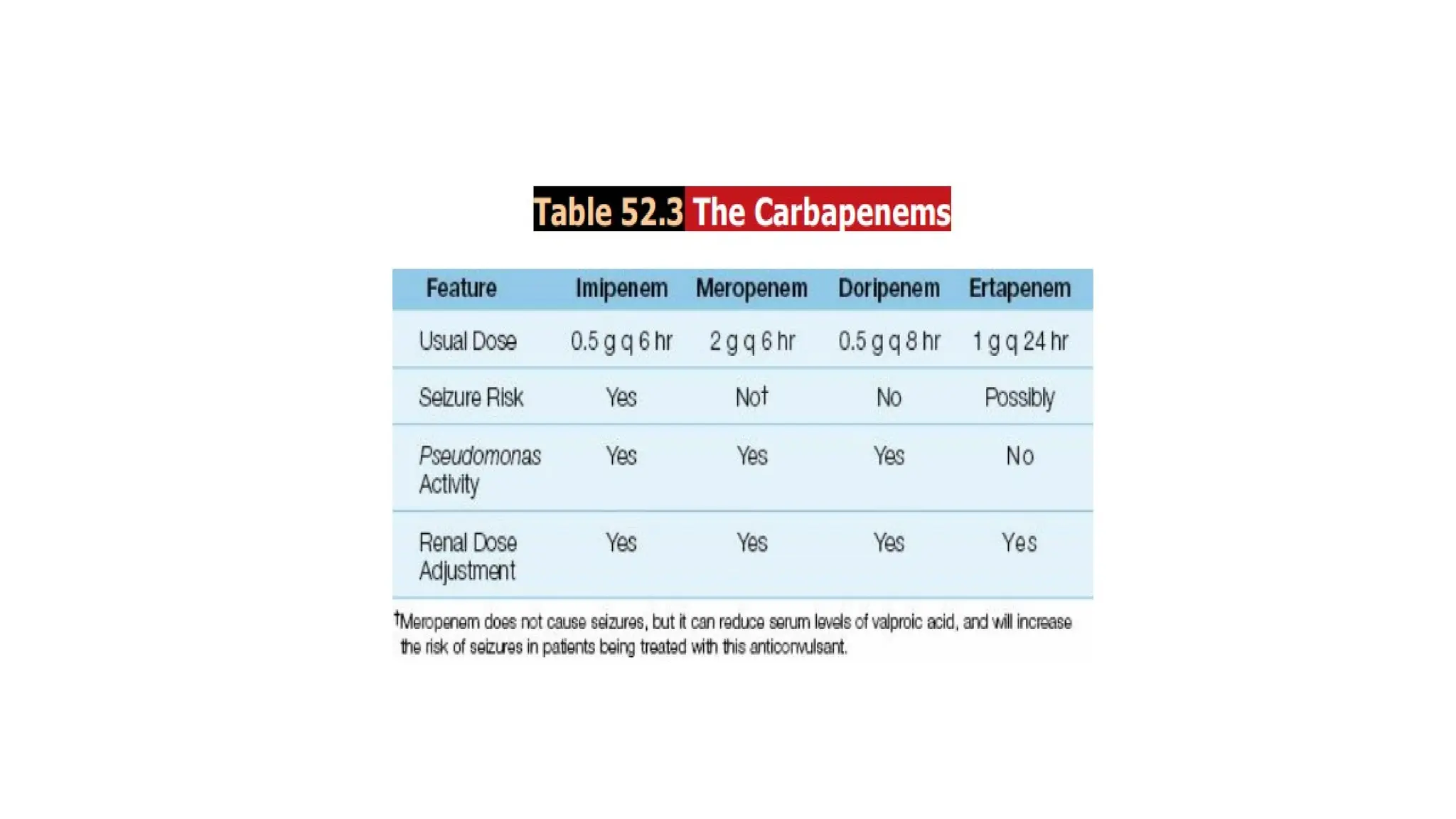
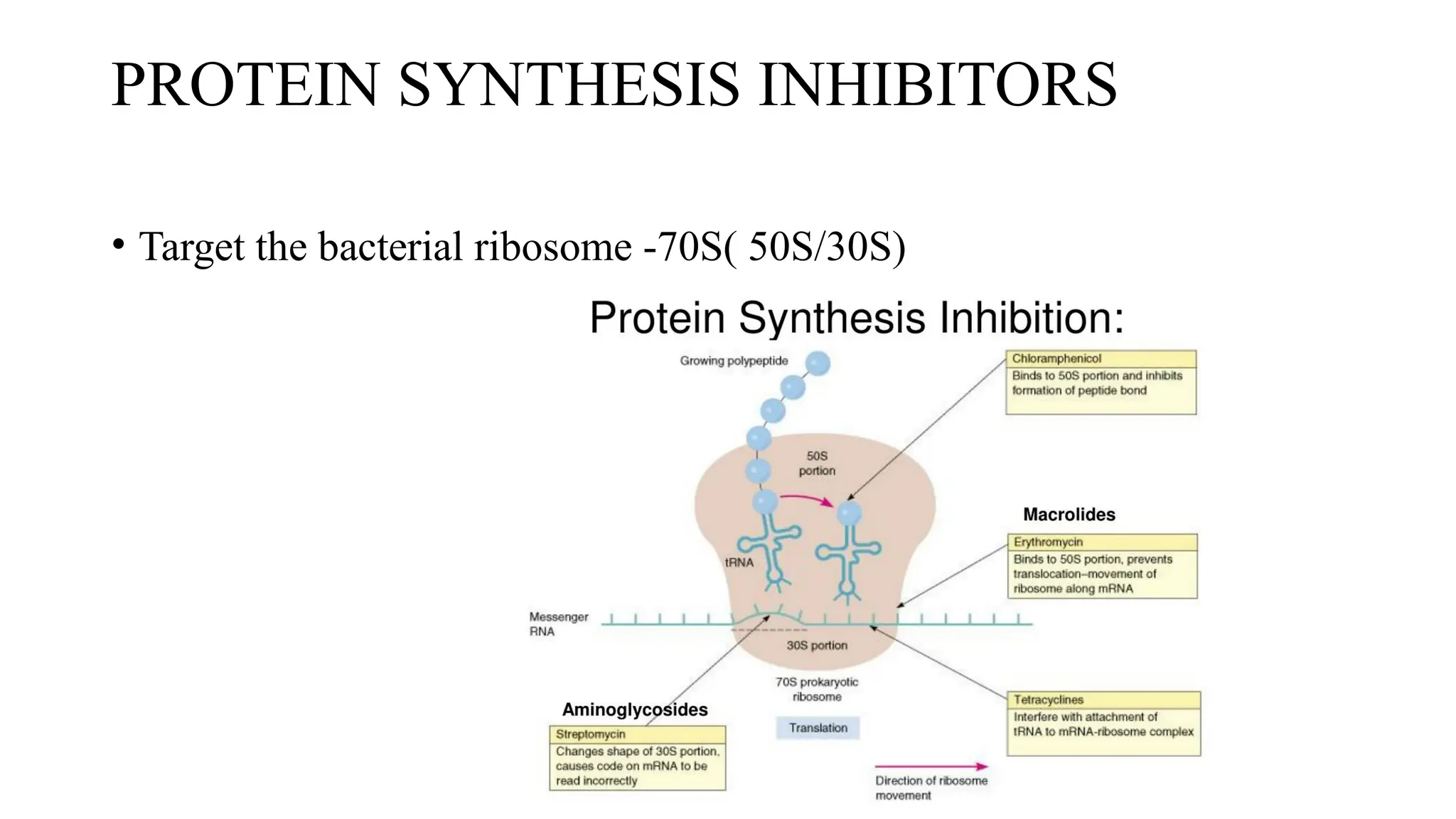
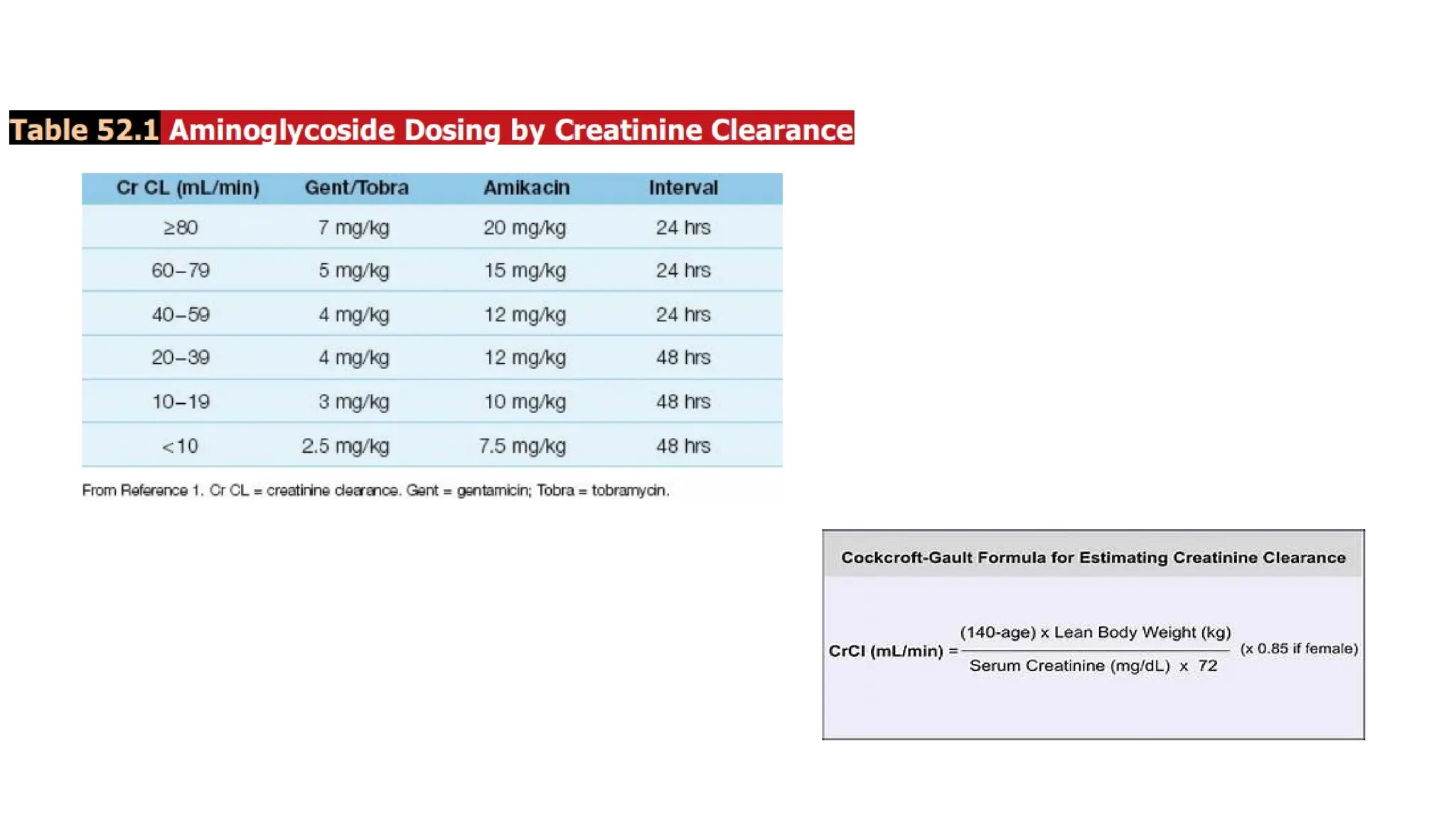
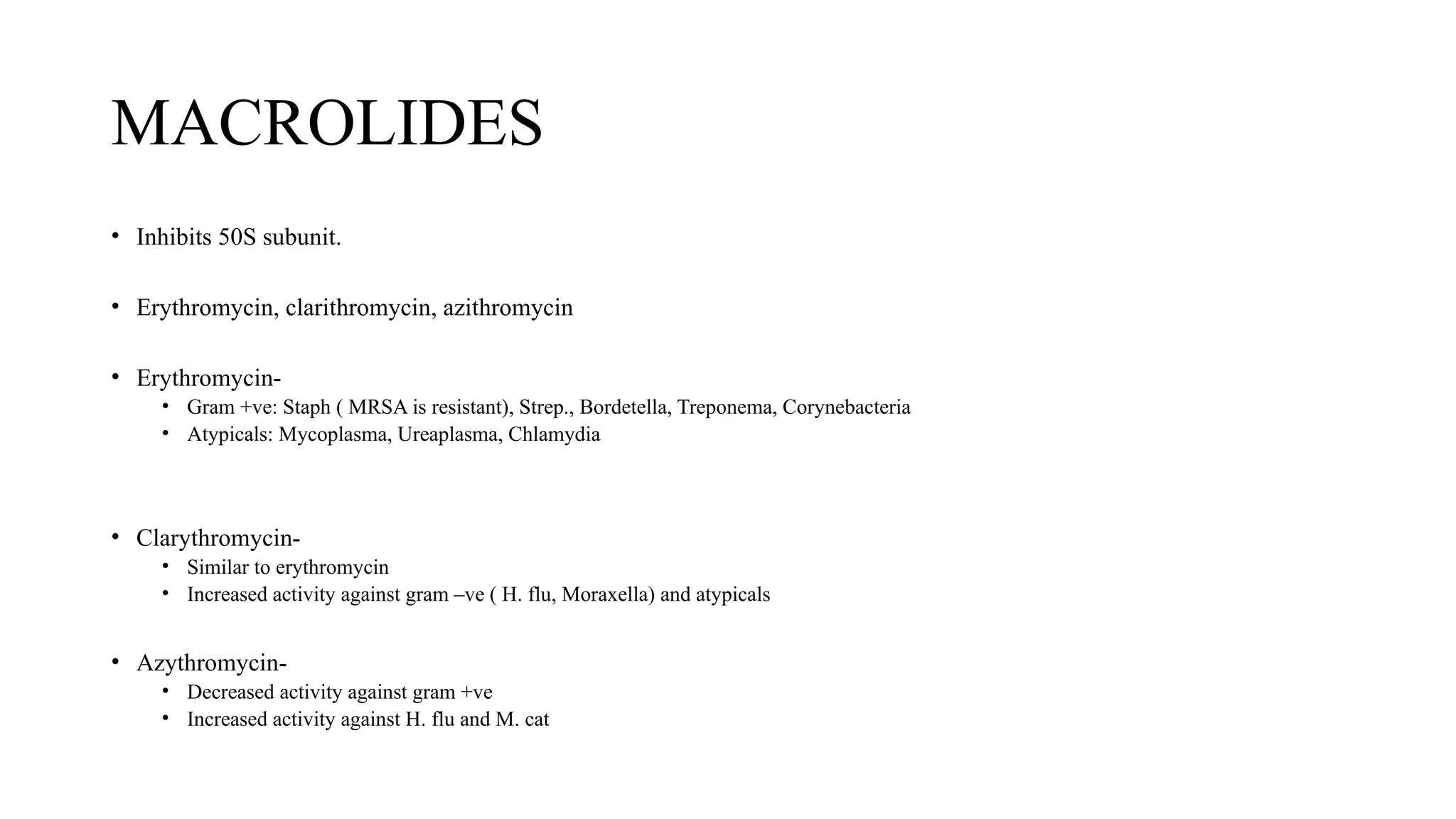
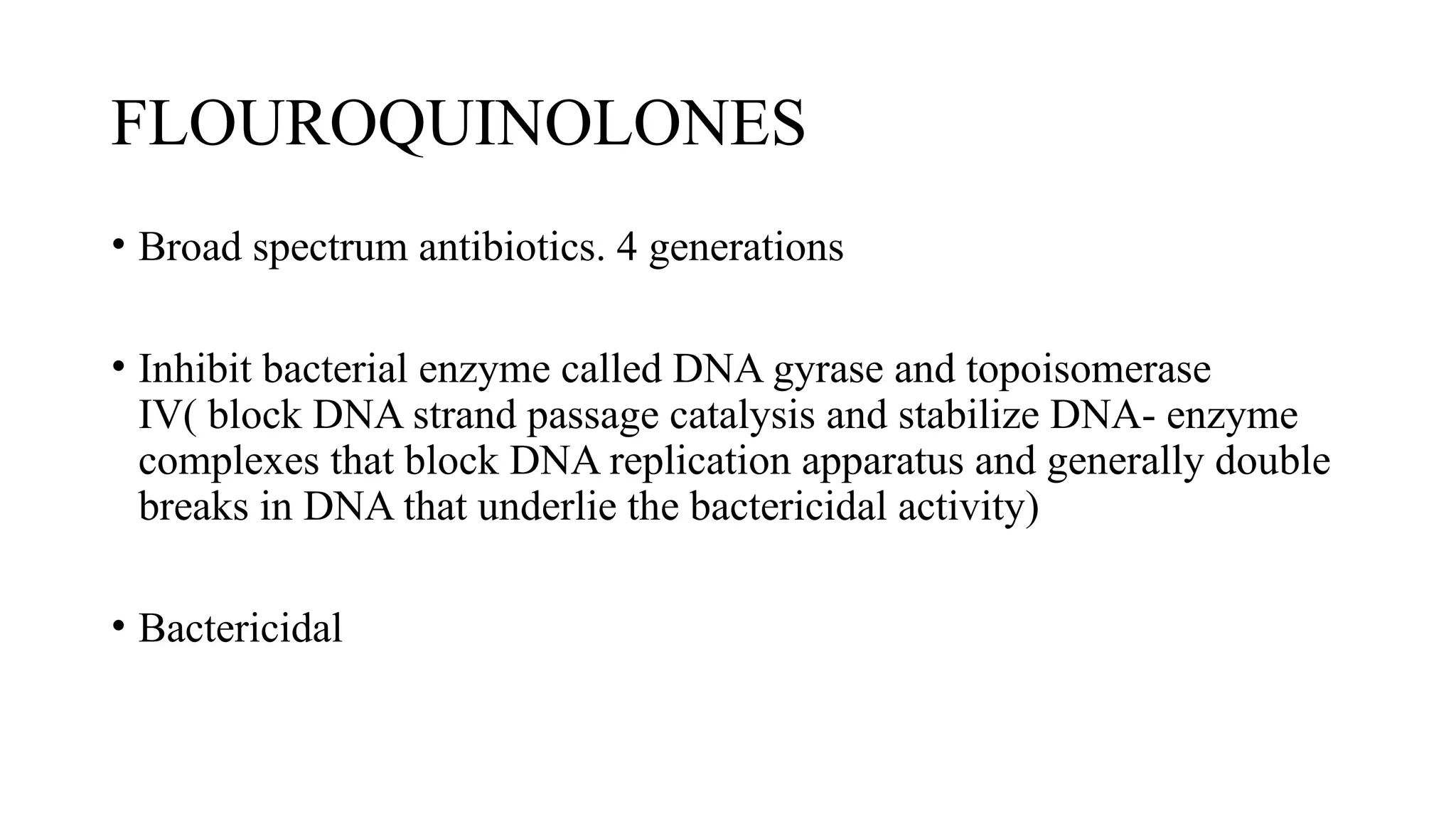
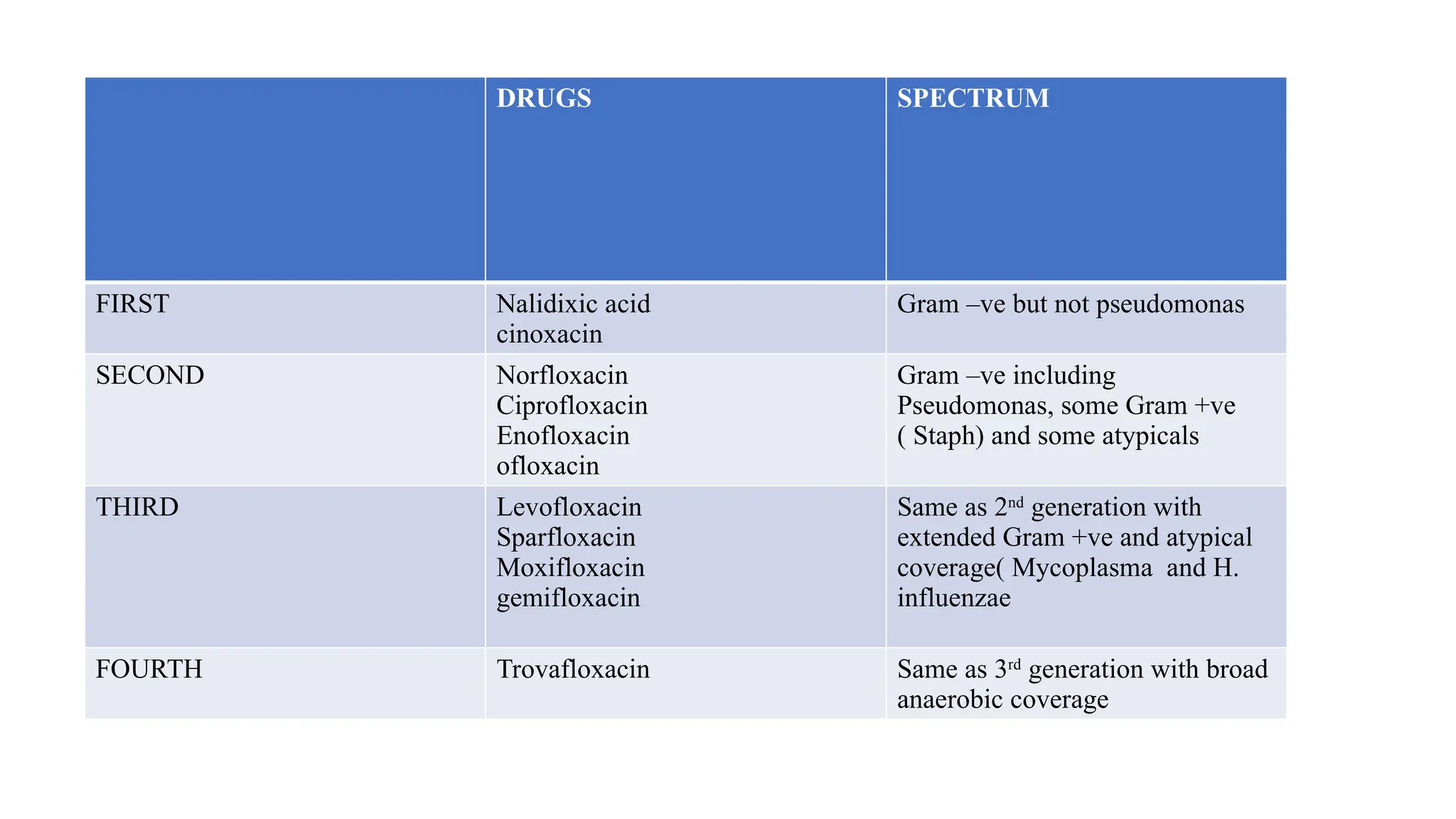
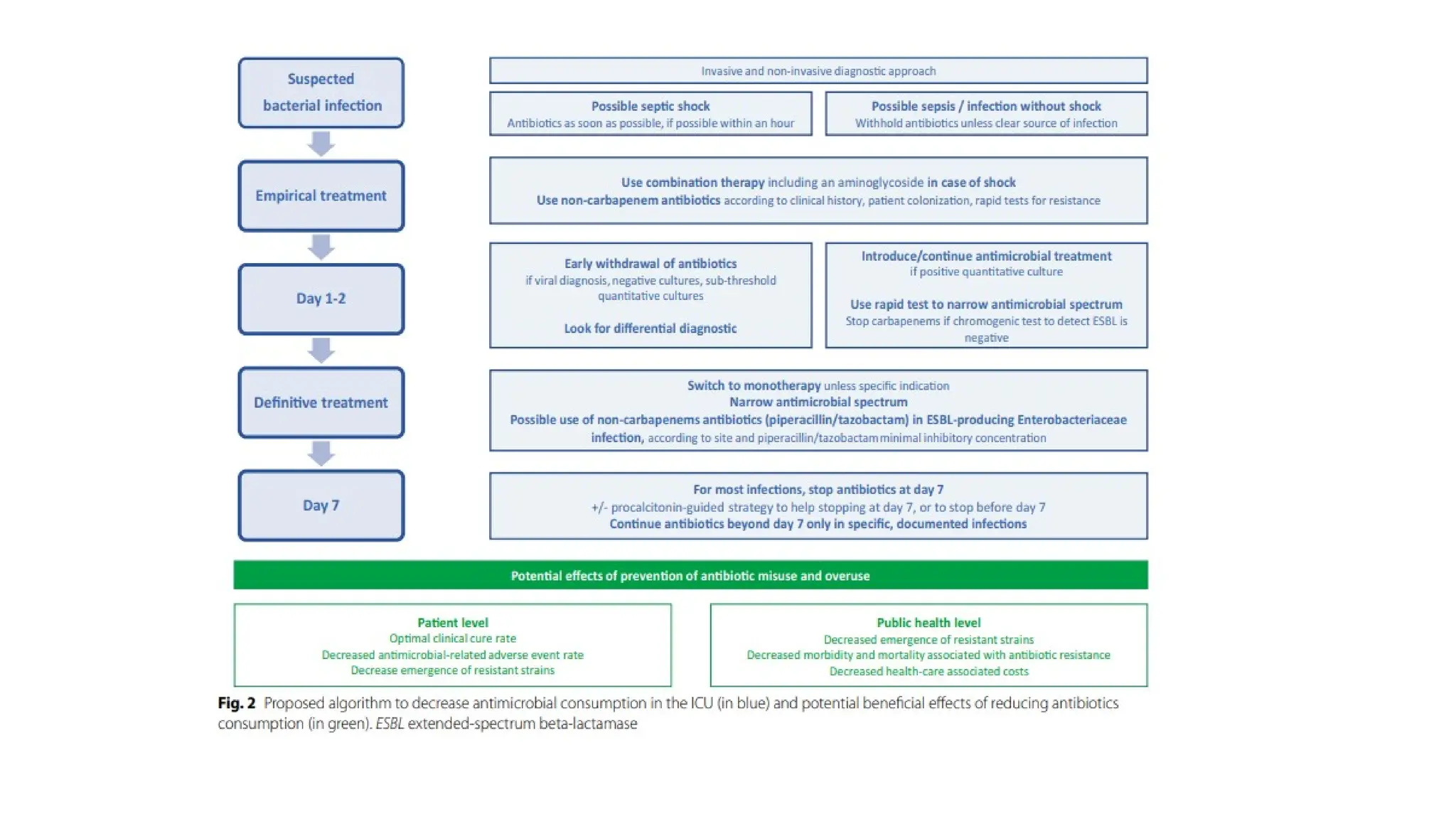
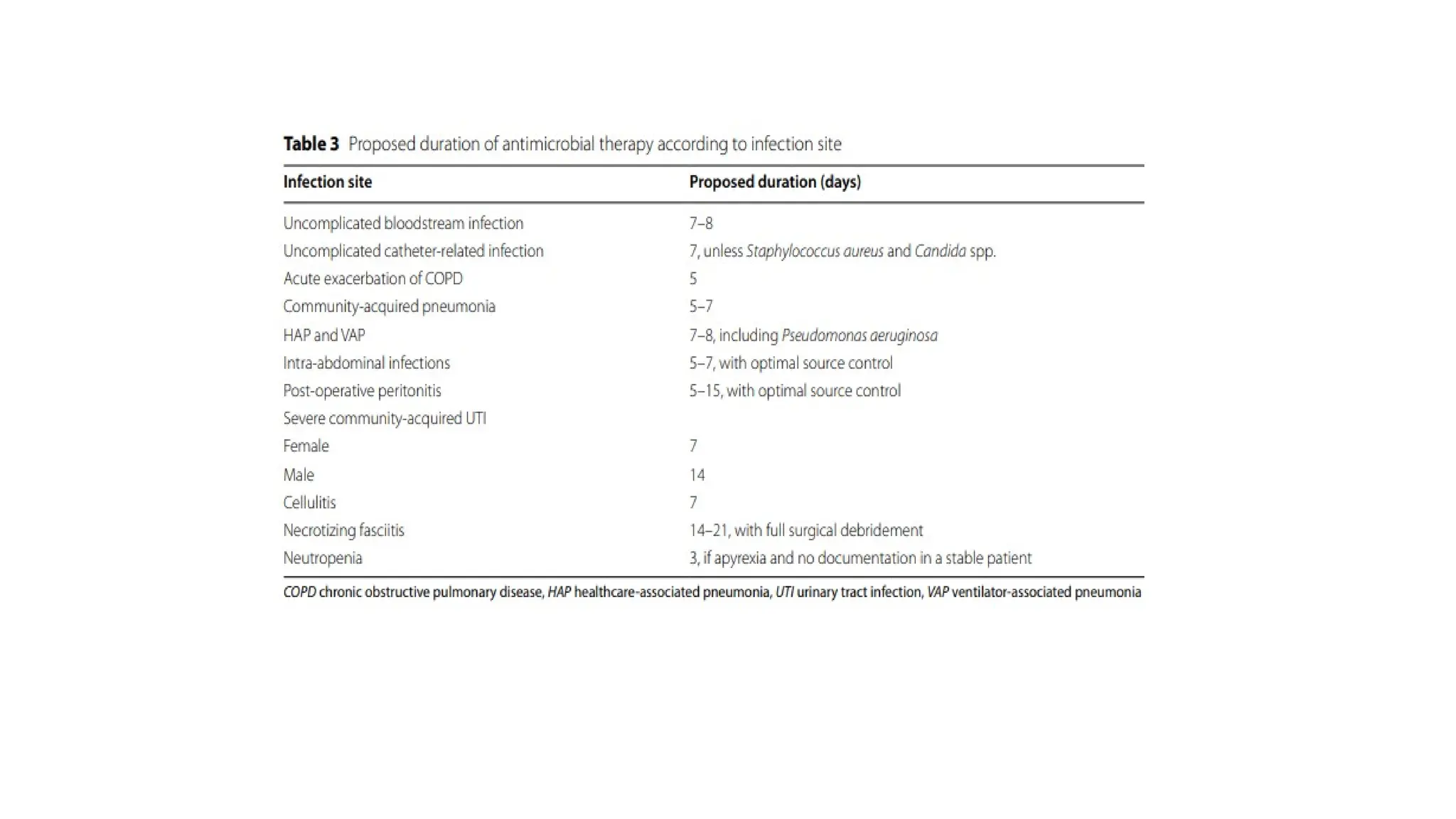
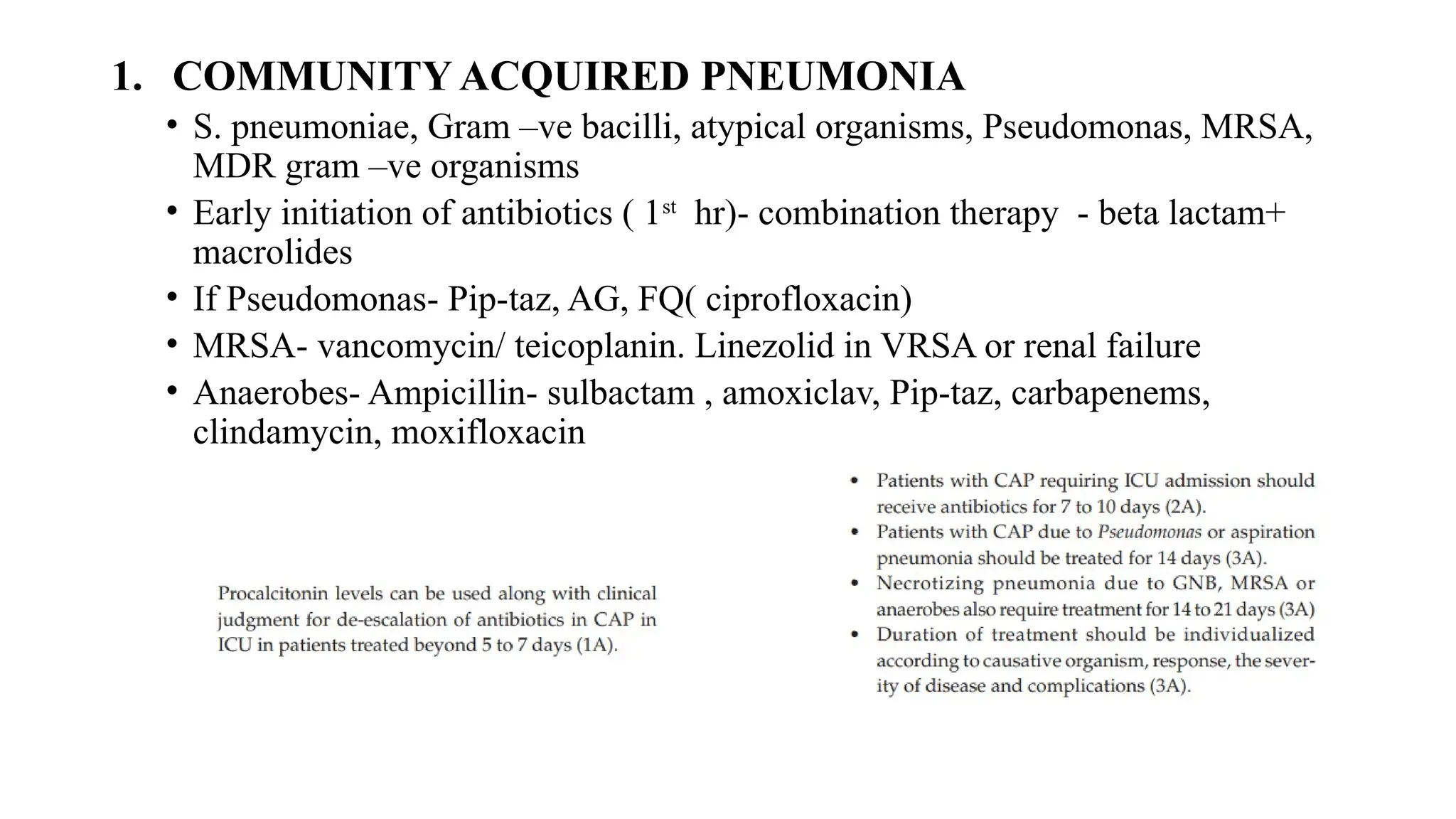
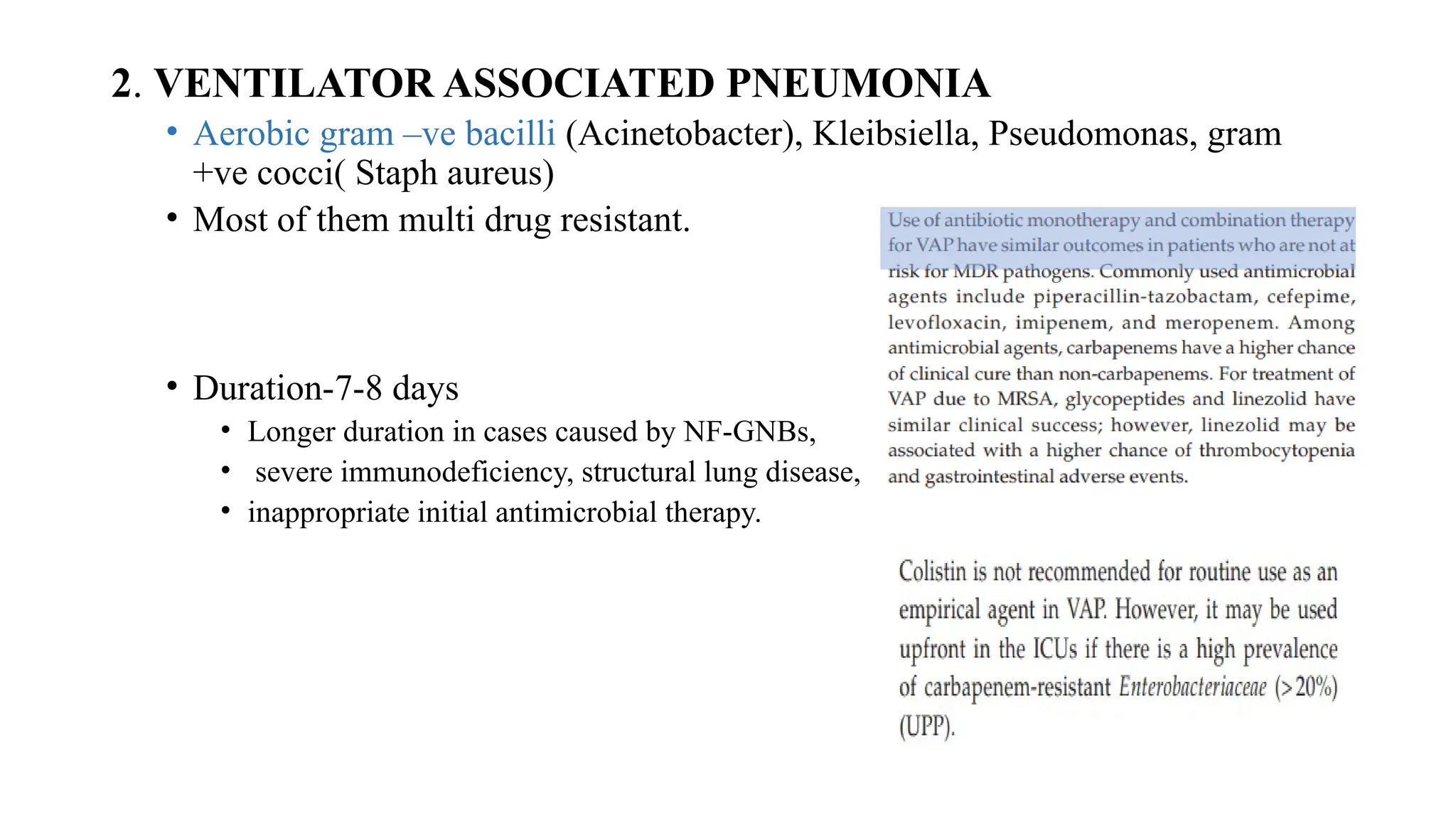
The document discusses the use of antibiotics in ICU settings, focusing on their classification, mechanisms of action, and the importance of antibiotic stewardship to minimize resistance. It emphasizes the need for rapid recognition and treatment of infections, alongside detailed guidelines for empirical therapy for various types of infections commonly encountered in critically ill patients. Key considerations include the pharmacokinetics and dynamics of antibiotics, resistance mechanisms, and best practices for optimizing antibiotic therapy.