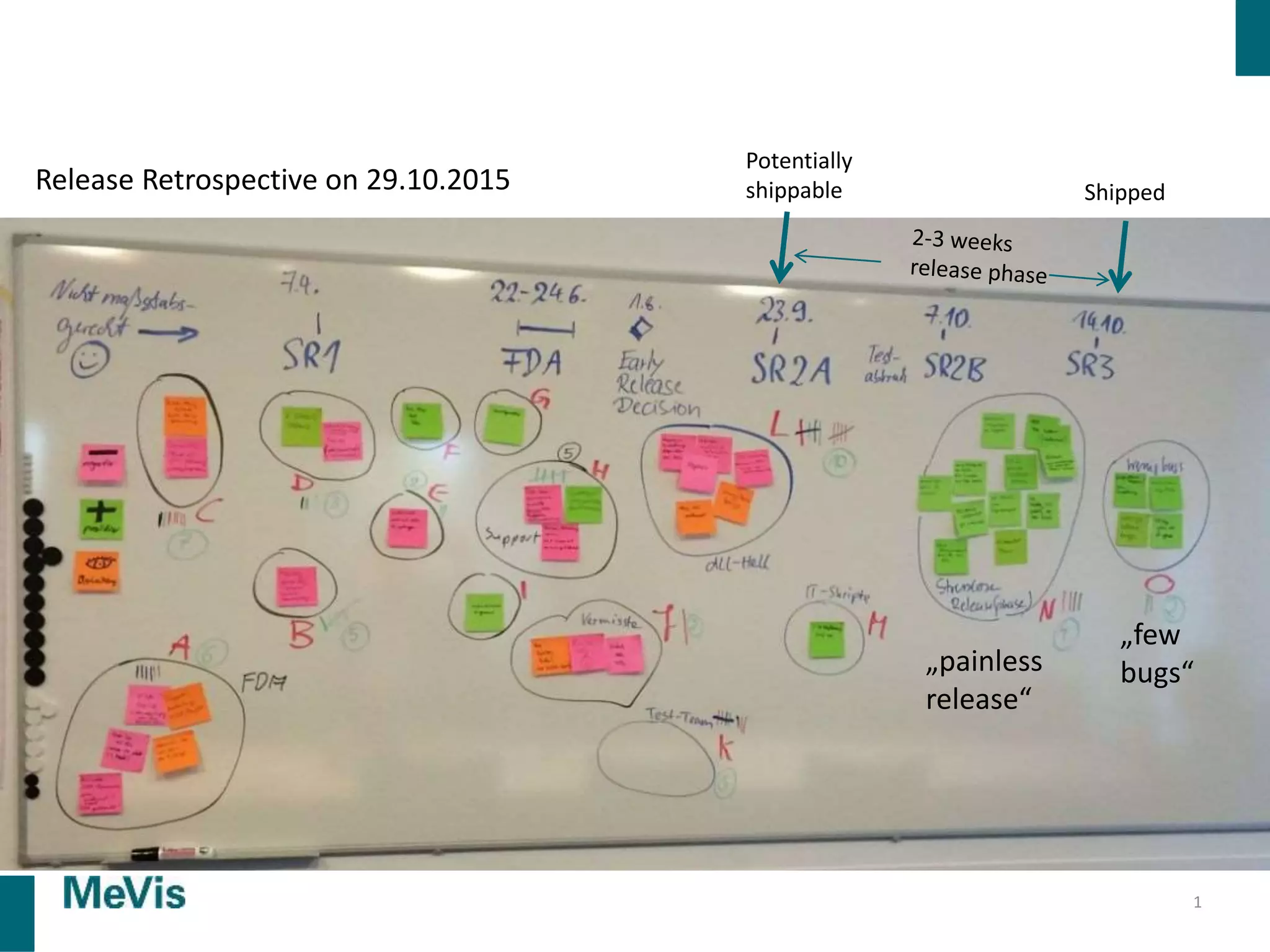
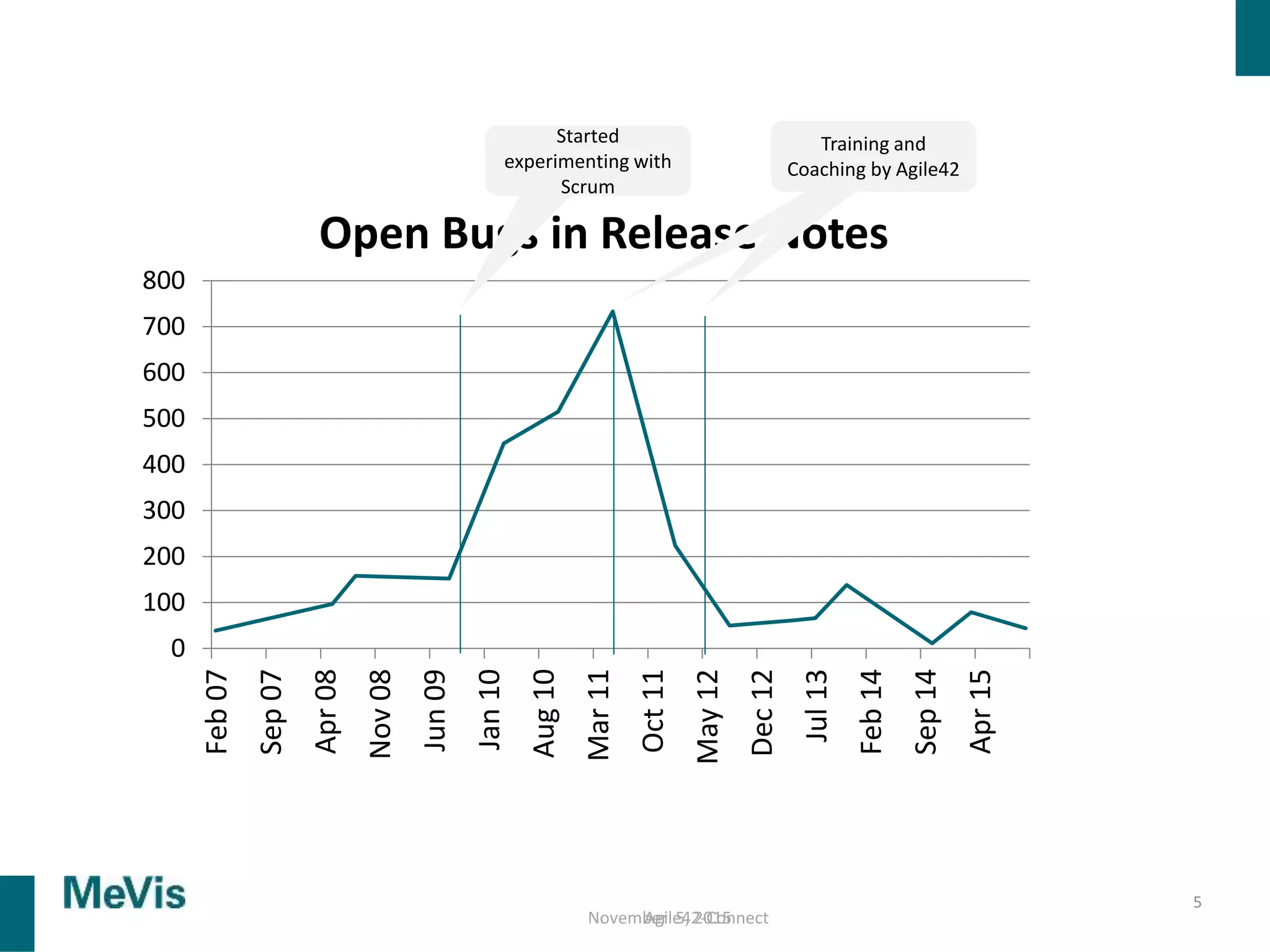
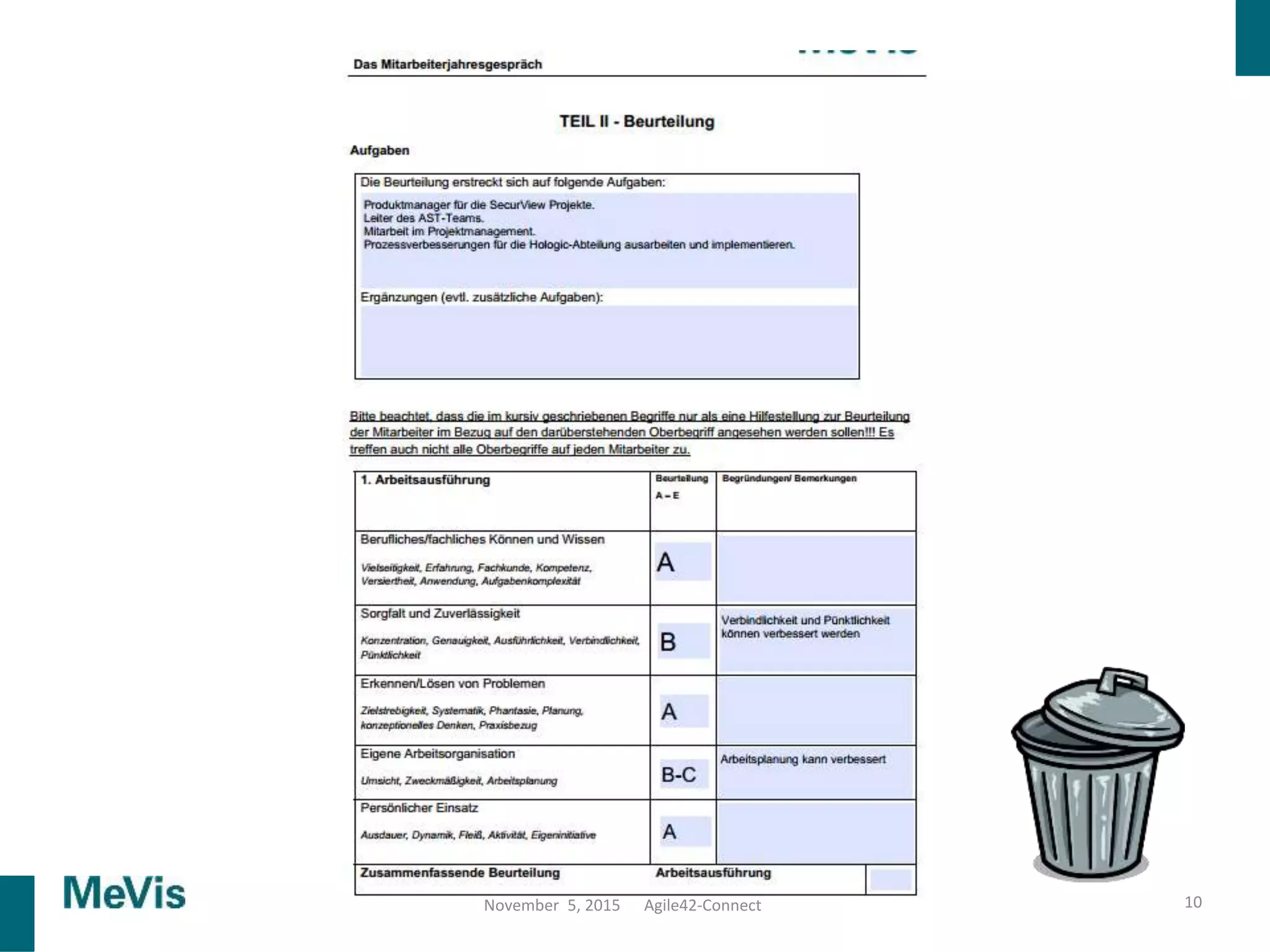
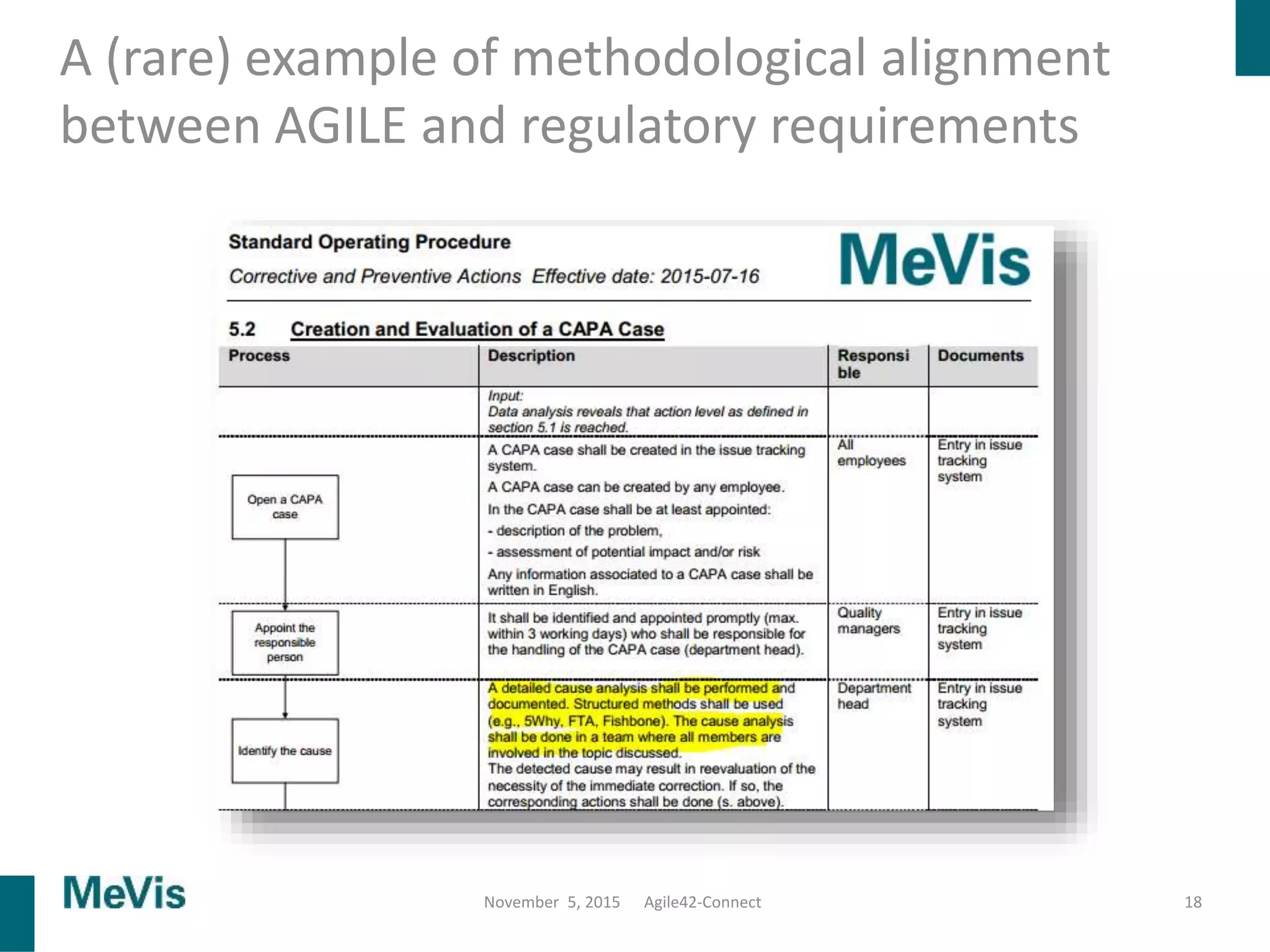
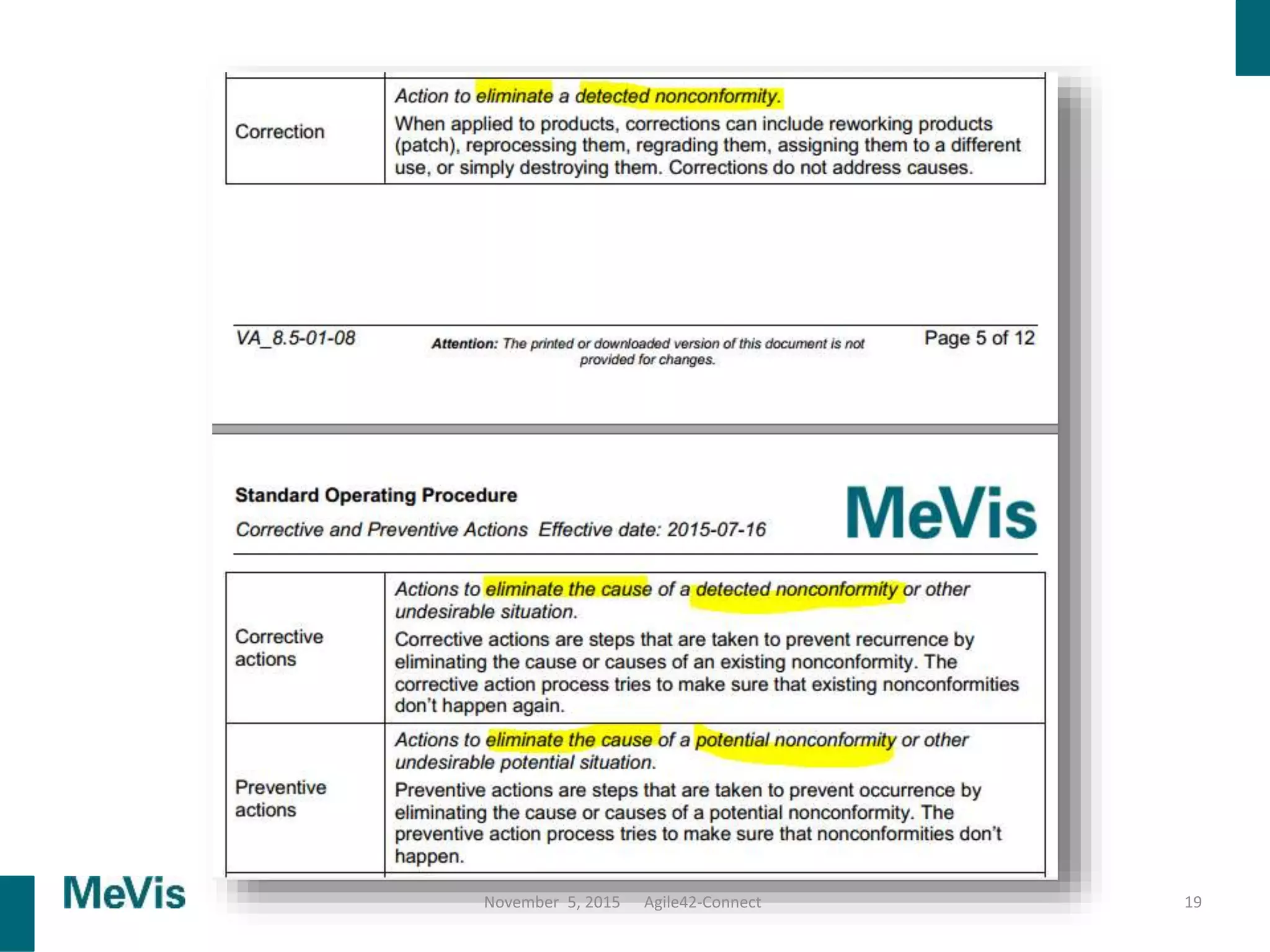
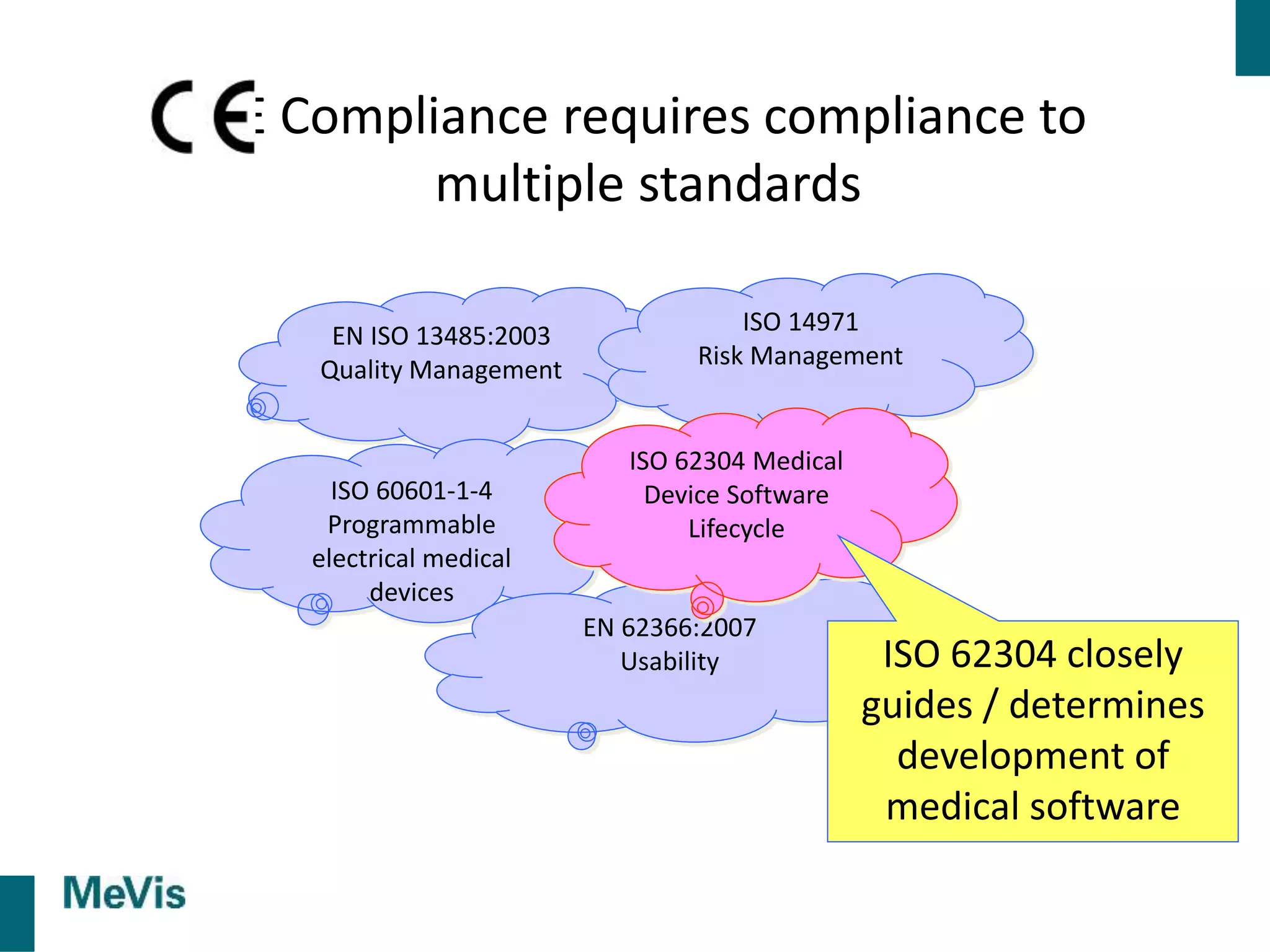
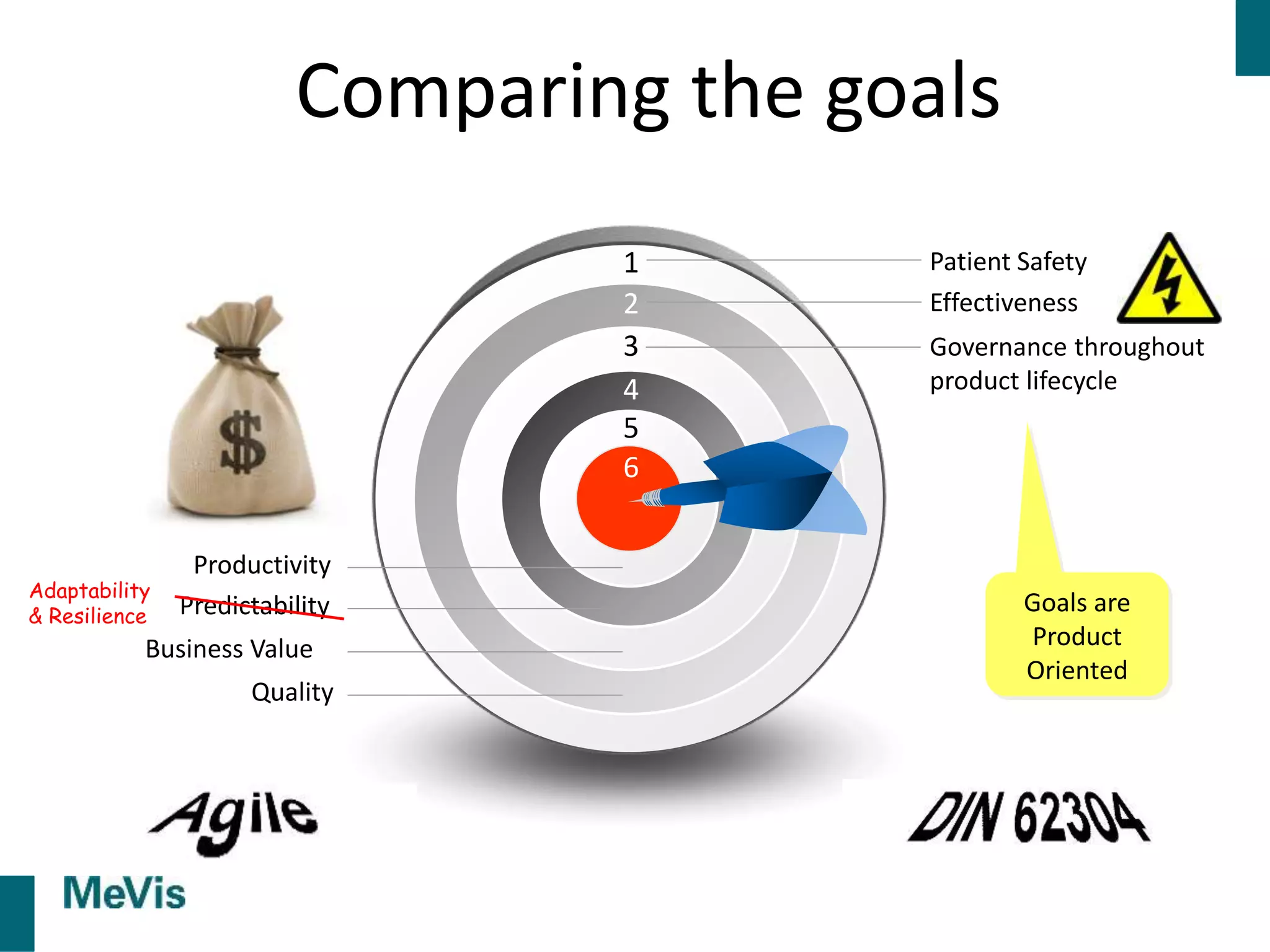
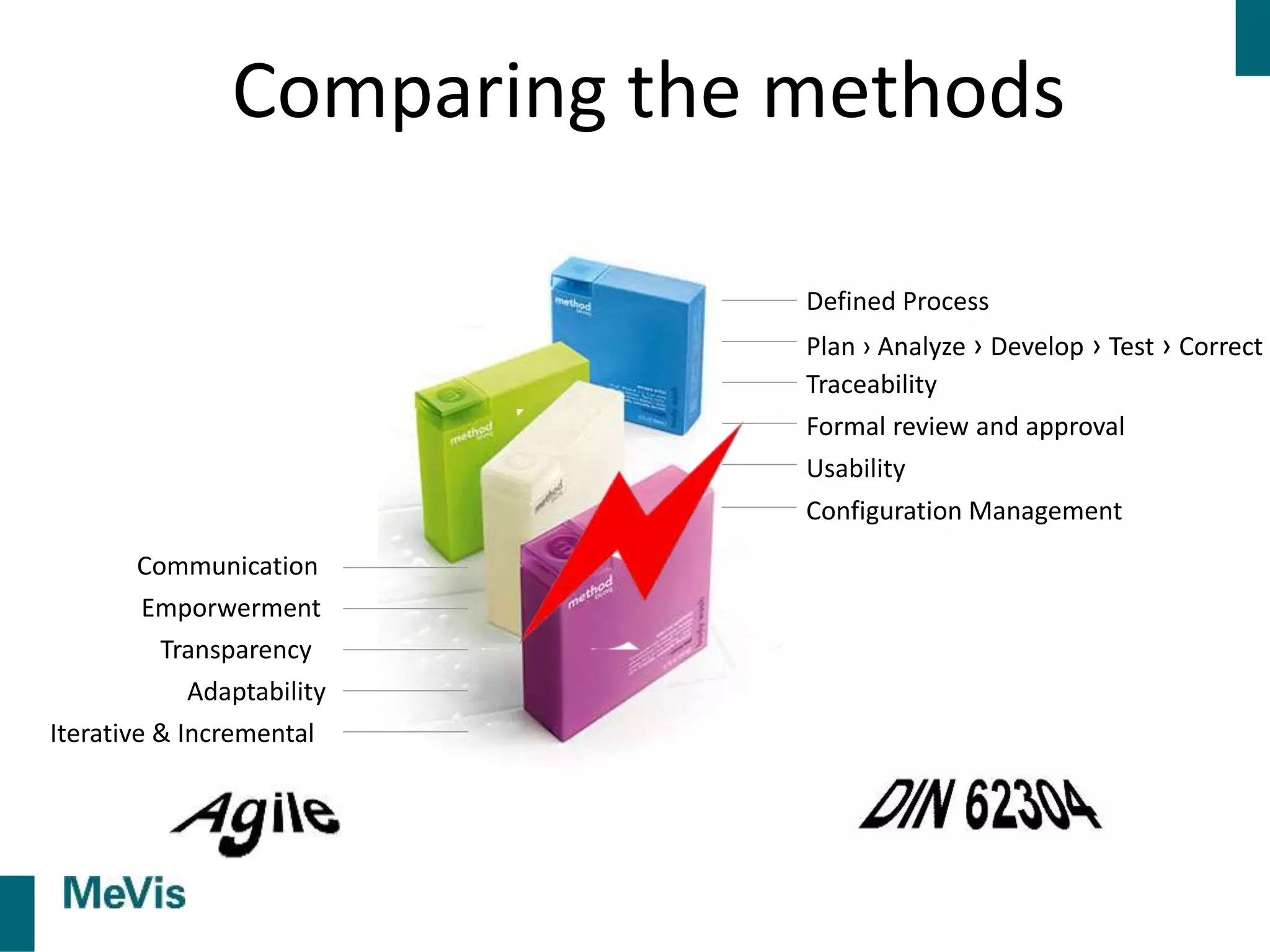
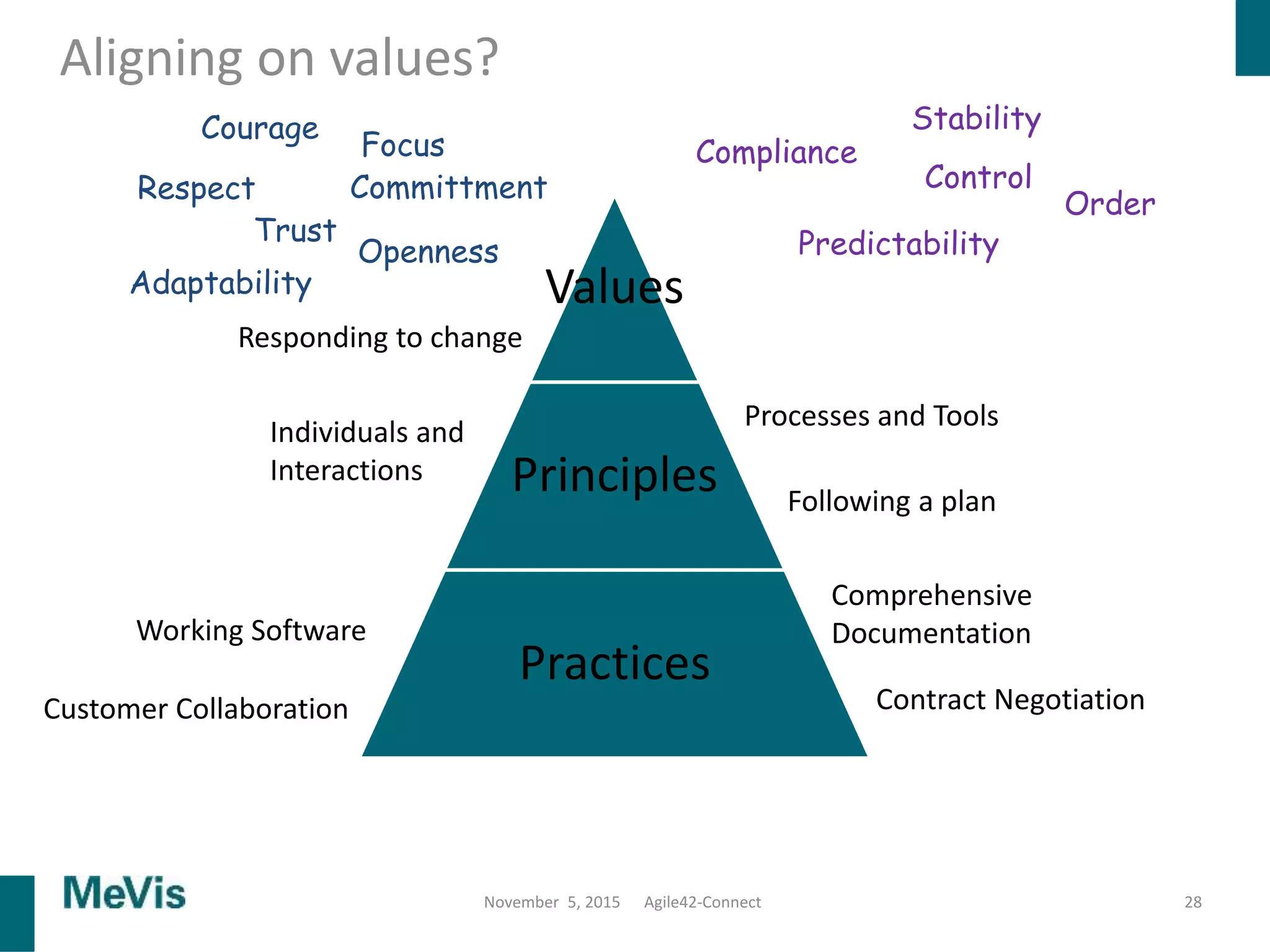
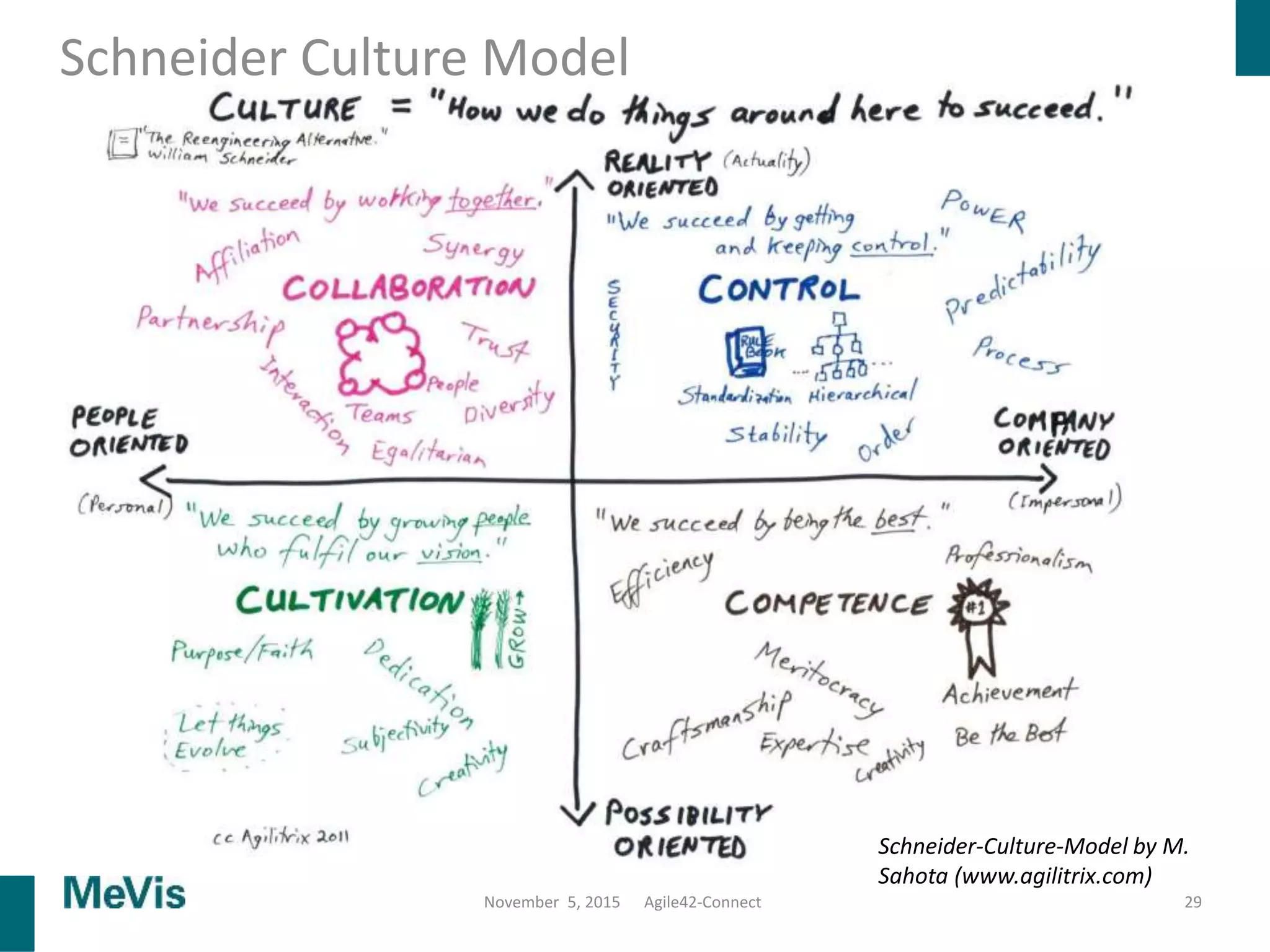
The document details a retrospective on agile transformation at Mevis Medical Solutions, highlighting their experience with implementing agile practices in a regulated environment. It discusses the alignment of agile values with regulatory requirements, the challenges faced, and the benefits realized, such as improved team collaboration and reduced bugs in releases. Lastly, it presents the importance of cultural changes and transparency within the organization to foster a successful agile framework.