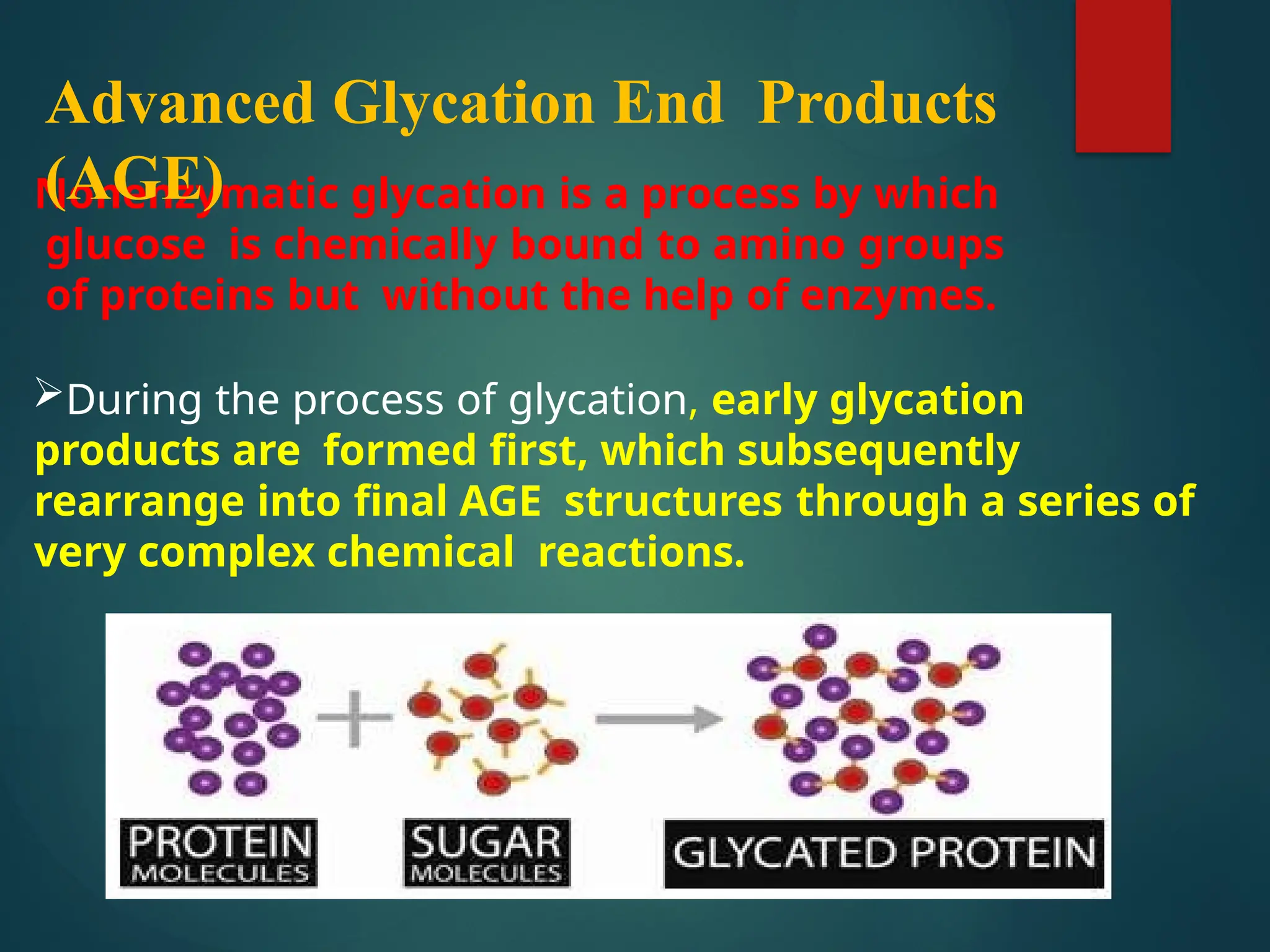
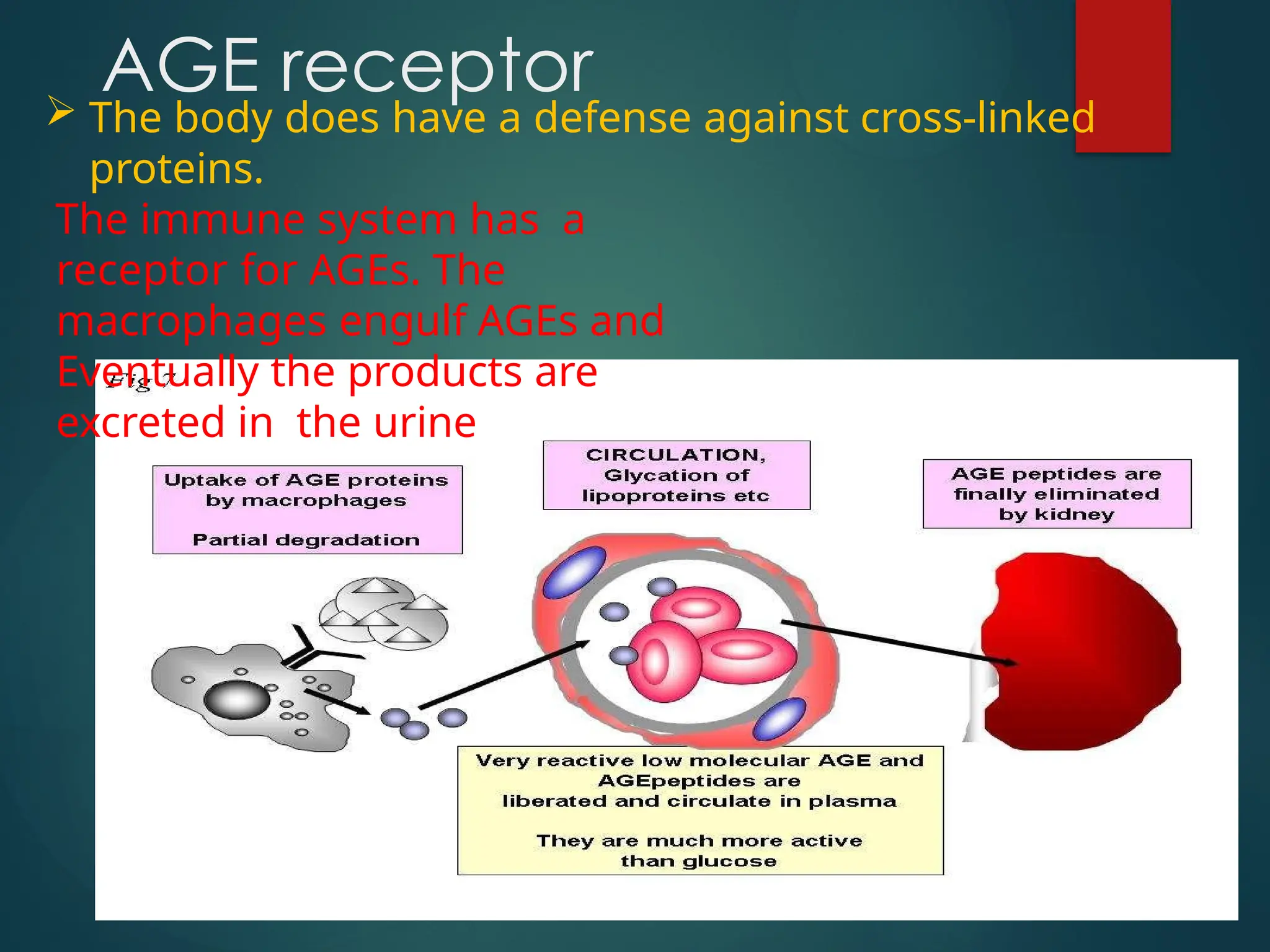
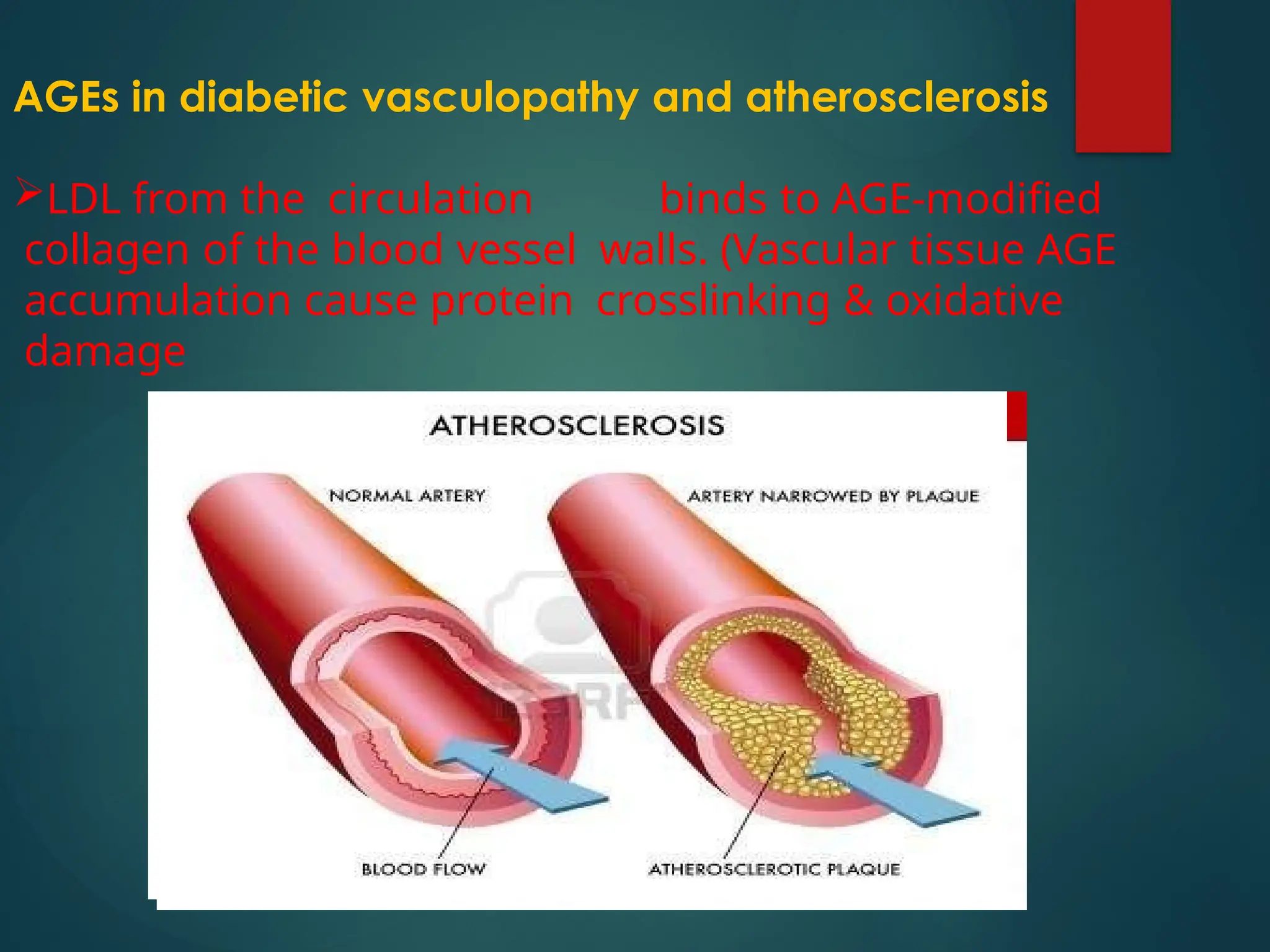
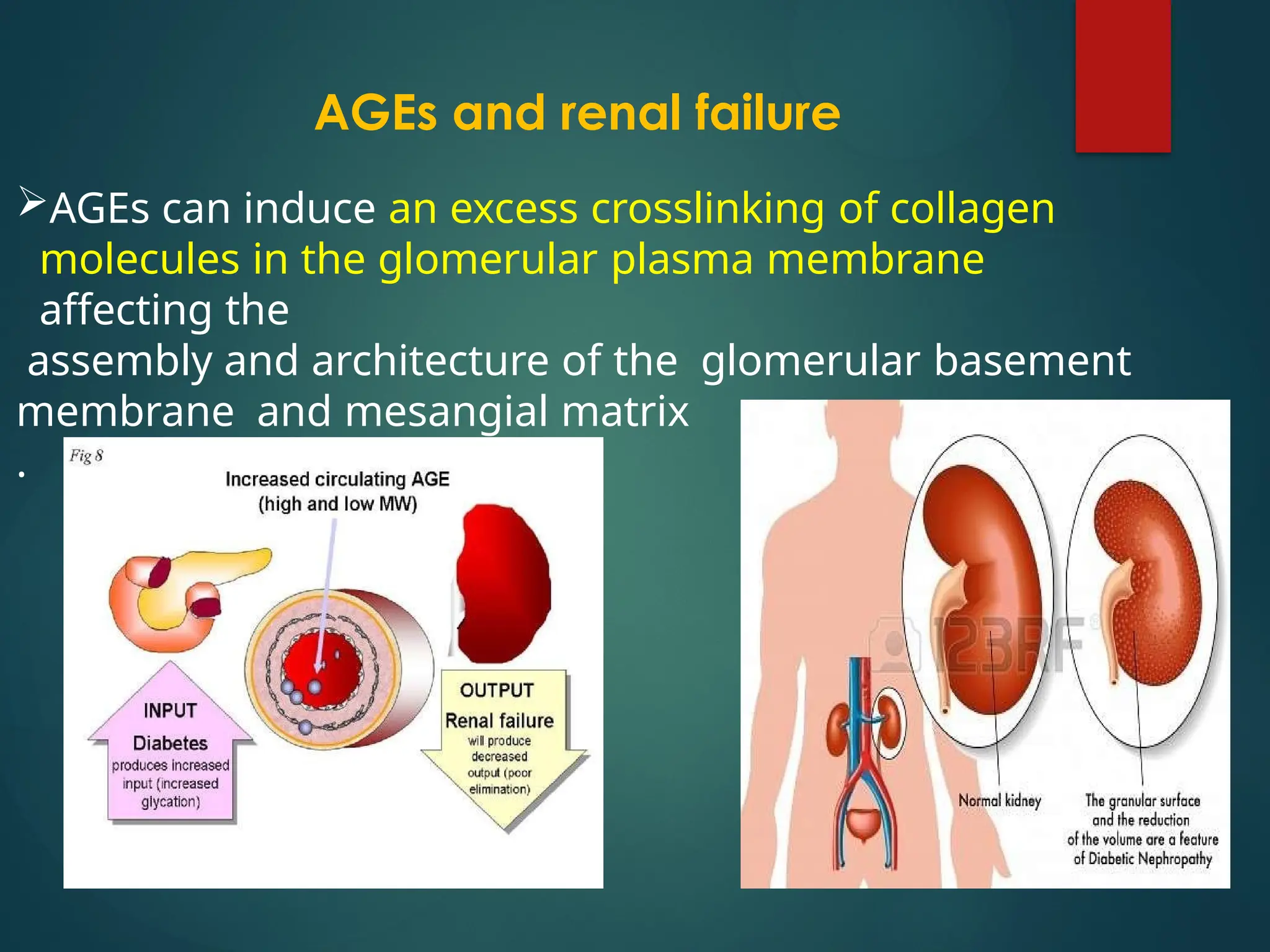
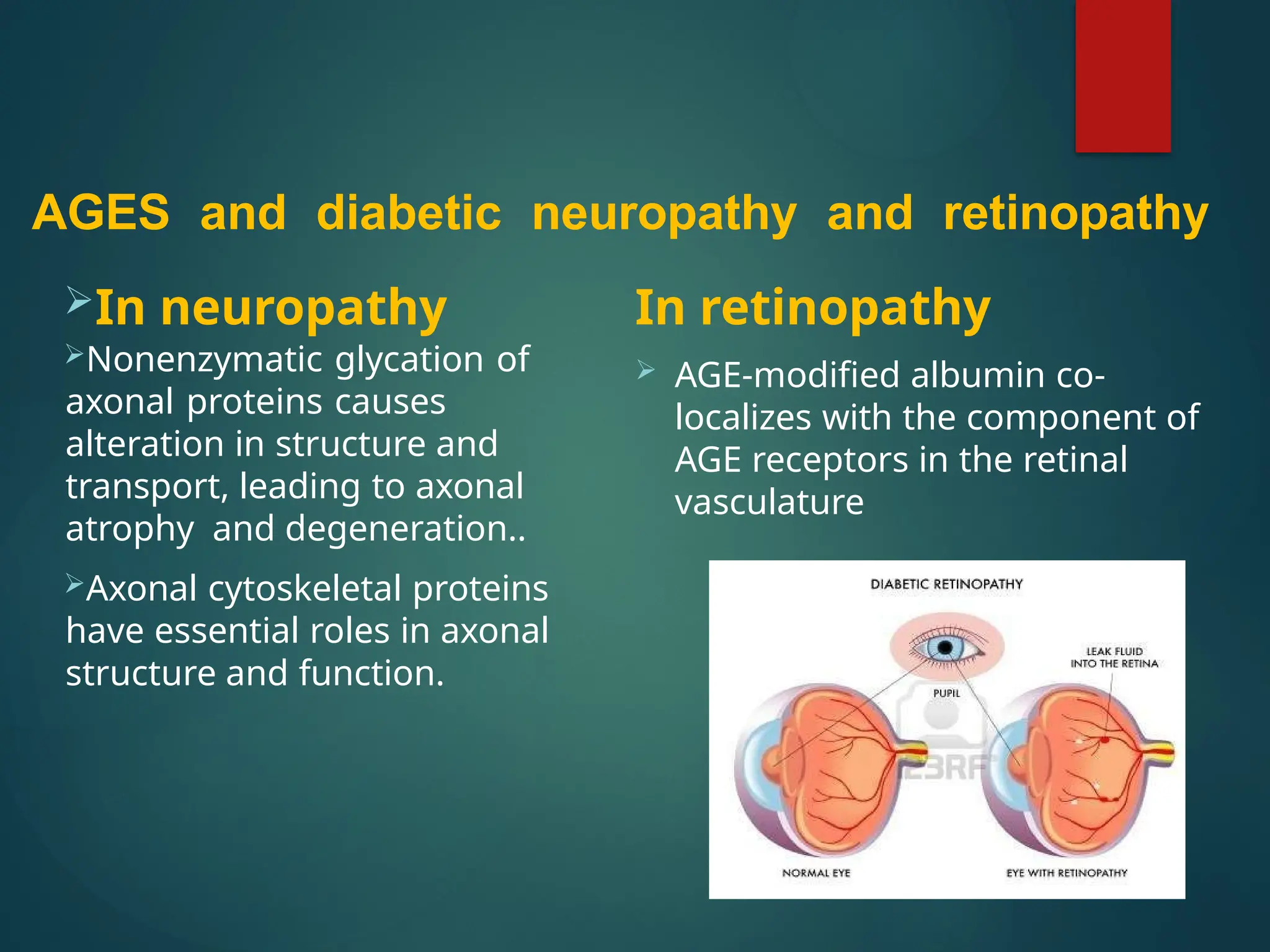
Advanced glycation end products (AGEs) result from the nonenzymatic glycation of proteins and contribute to tissue damage, particularly in conditions like diabetes. AGEs can originate from both external sources, such as diet and tobacco smoke, and internal physiological processes, leading to complications like diabetic neuropathy, retinopathy, and vascular issues. The body has defense mechanisms against AGEs, including immune responses that help to clear these harmful compounds.