i3 Health is pleased to make the speaker slides from this activity available for use as a non-accredited self-study or teaching resource.
Schizophrenia impacts millions globally, presenting with diverse positive, negative, and cognitive symptoms that significantly affect patients' lives. Current treatments often fall short across these domains, underscoring the need for innovative approaches. Dr. Christoph U. Correll explores the latest evidence-based strategies, including new and emerging therapeutic agents, how to implement patient-centered approaches and how shared decision-making will optimize outcomes in this slide presentation.
Statement of Need
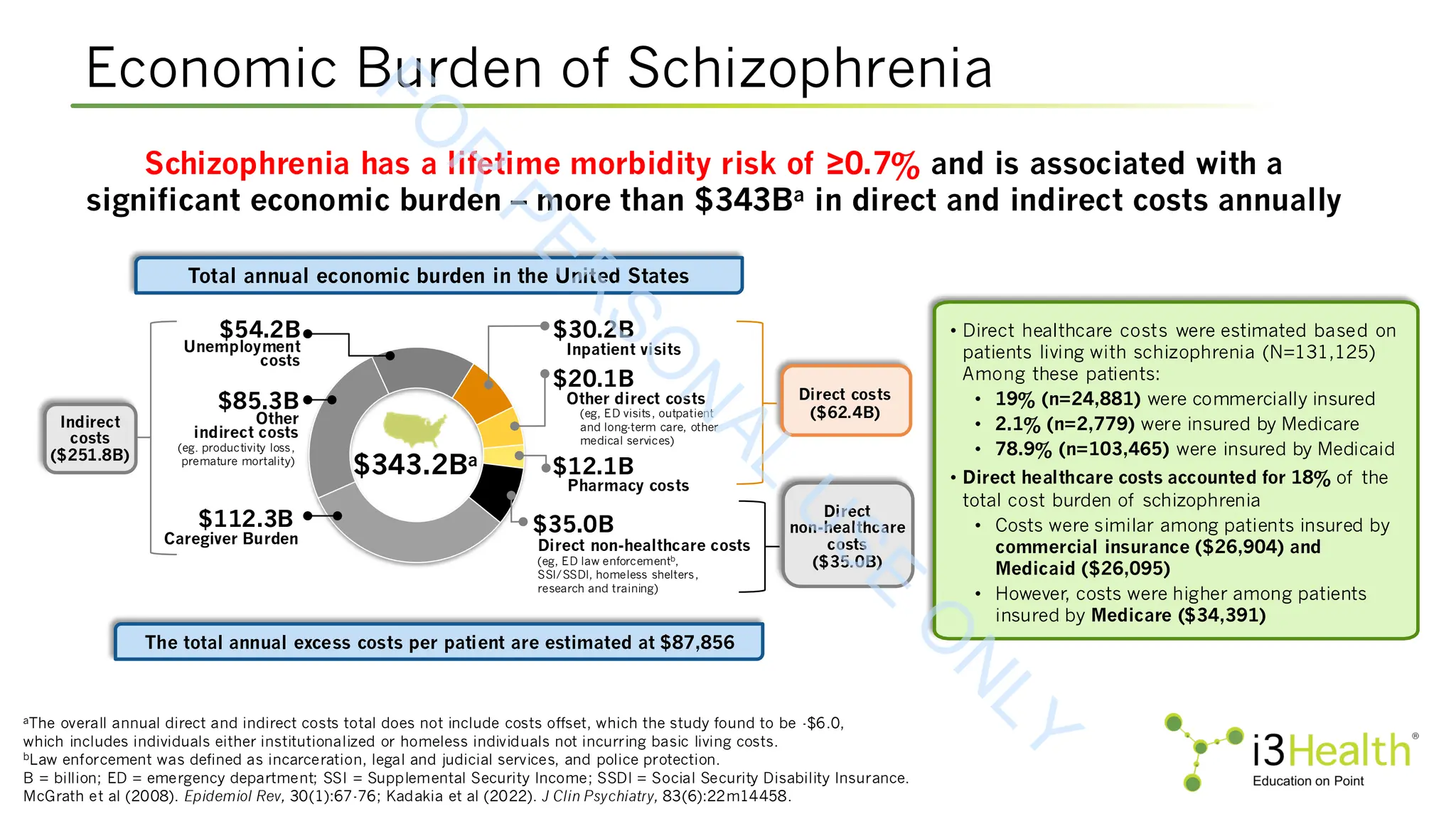
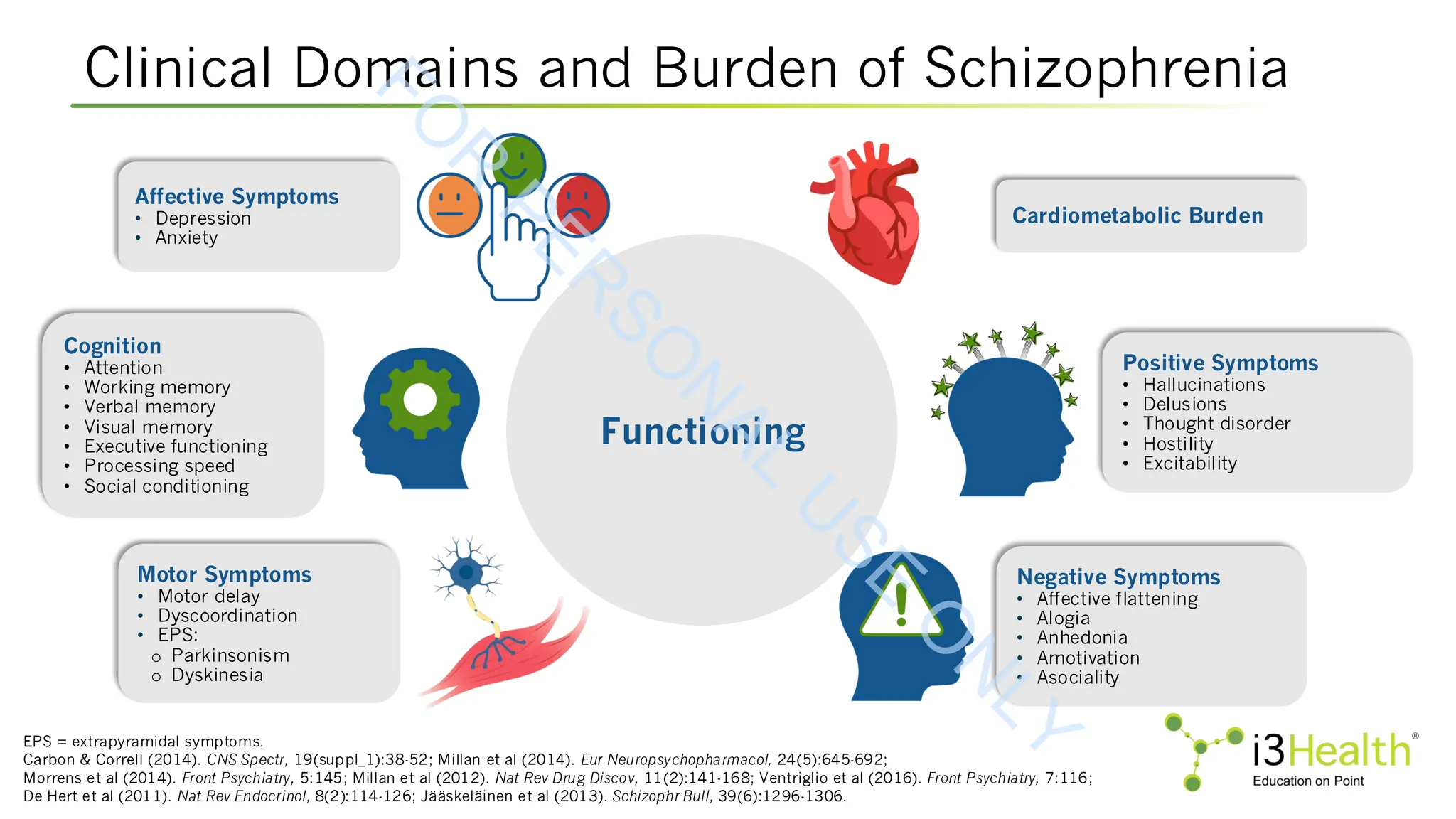
Schizophrenia is a complex psychiatric disorder that affects approximately 24 million people worldwide. The disease is characterized by heterogenous symptom trajectories which drastically impact patients’ quality of life and outcomes (Kinon et al, 2024). Schizophrenia includes three primary symptom domains: positive symptoms, such as hallucinations, delusions, illogical thought and behavior changes, hyperactivity, and thought disorders; negative symptoms, such as apathy, lethargy, and social withdrawal; and cognitive symptoms, such as difficulty in abstract thinking, poor attention, disorientation, stereotyped thinking, and conceptual disorganization (Agius et al, 2024; Kinon et al, 2024; McCutcheon et al, 2023). Currently, the approved treatments for schizophrenia are limited in their efficacy across symptom domains (Kinon et al, 2024). Therefore, it is crucial for the care team to remain up to date not only on advances in new and emerging therapeutic agents, but also on patient-centered approaches that incorporate shared decision making in order to optimize outcomes for their patients.
Target Audience
Psychiatrists, psychiatric nurse practitioners, psychiatric physician assistants/associates, psychiatric-mental health nurses, pharmacists, and other healthcare professionals (HCPs) involved in the treatment of patients with schizophrenia.
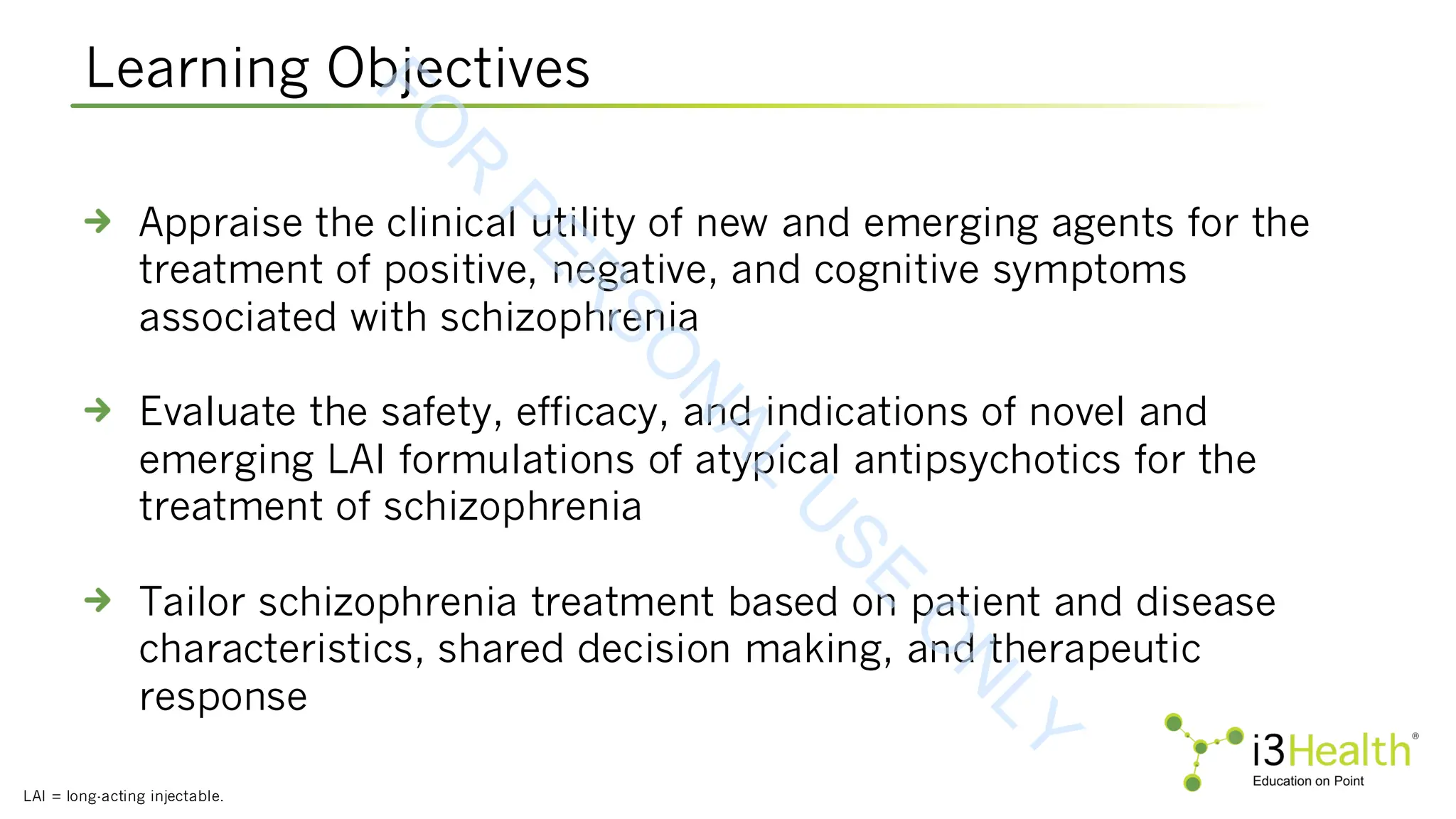
Learning Objectives
Appraise the clinical utility of new and emerging agents for the treatment of positive, negative, and cognitive symptoms associated with schizophrenia
Evaluate the safety, efficacy, and indications of novel and emerging LAI formulations of atypical antipsychotics for the treatment of schizophrenia
Tailor schizophrenia treatment based on patient and disease characteristics, shared decision making, and therapeutic response
BIO
Christoph U. Correll, MD, is a Professor of Psychiatry and Molecular Medicine at Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in New York. He is also a Professor and Chair of the Department of Child and Adolescent Psychiatry at Charité – University Medicine in Berlin, Germany. Dr. Correll’s research focuses on early identification and treatment of children and adults with severe mental illness, including schizophrenia and other psychotic disorders.




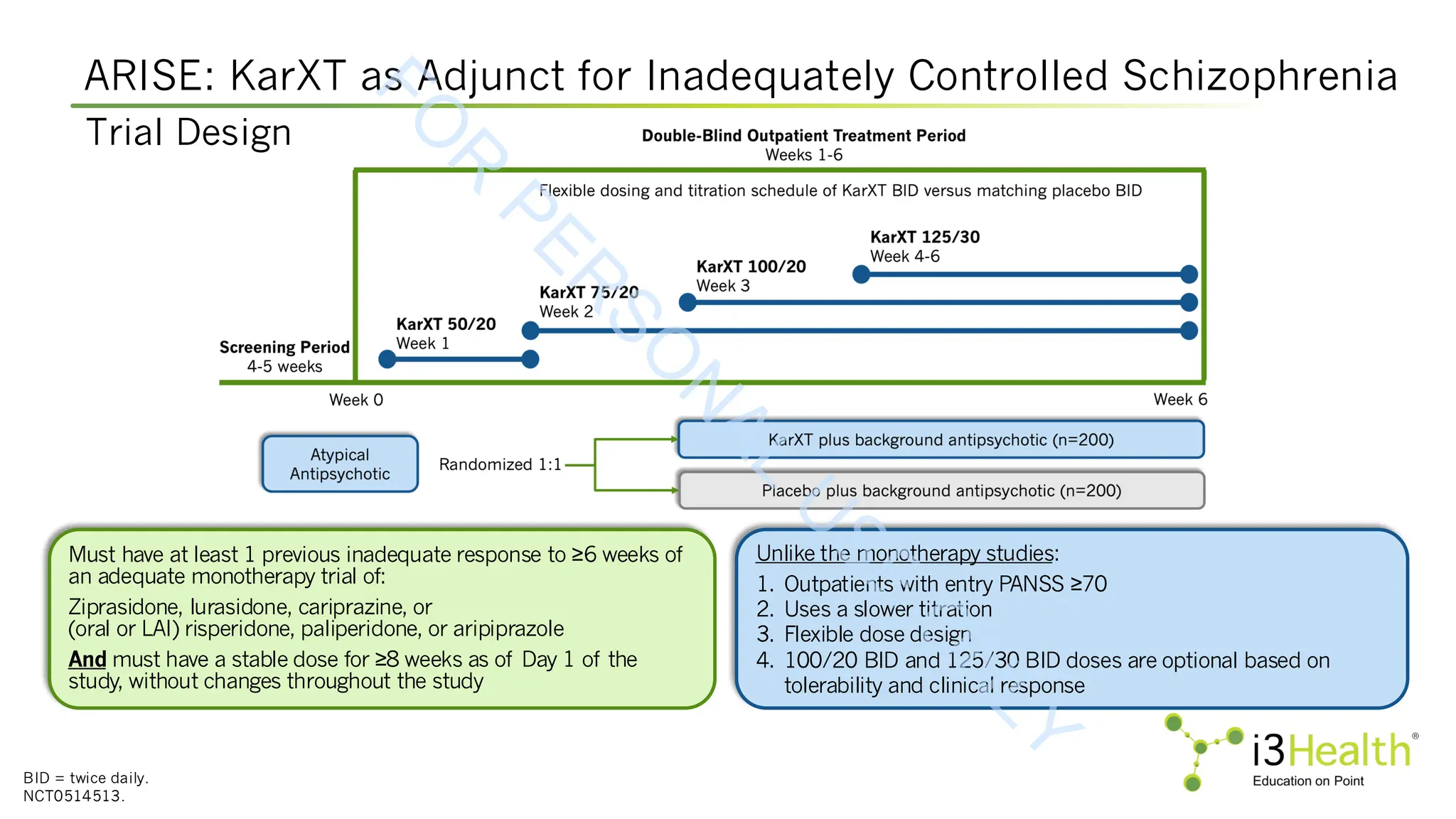
![ARISE Trial: Xanomeline-Trospium (XT) vs Placebo
APD = antipsychotic background drug; PSP = Personal and Social Performance Scale; mITT = modified intent-to-treat; SE = standard error;
LSMD = least squares mean difference.
aP value is nominal, not adjusted for multiplicity.
Bristol Myers Squibb, 2025 [https://news.bms.com/news/details/2025/Bristol-Myers-Squibb-Announces-Topline-Results-from-Phase-3-ARISE-Trial-
Evaluating-Cobenfy-xanomeline-and-trospium-chloride-as-an-Adjunctive-Treatment-to-Atypical-Antipsychotics-in-Adults-with-Schizophrenia/default.aspx].
LS Mean Change From Baseline to Week 6 in PANSS, PSP, and CGI-S
Non-risperidone group includes paliperidone, aripiprazole, ziprasidone, lurasidone and cariprazine.
Outcome XT + APD Placebo + APD LSMD (95% CI) P Value
mITT Population, N 190 196
Primary Endpoint
Change in PANSS
Total Score (SE)
-14.3 (1.01) -12.2 (0.98) -2.0 (-4.5, 0.5) 0.11
Key Secondary Endpoint
Change in PSP
Score (SE)
5.3 (0.75) 5.9 (0.73) -0.6 (-2.4, 1.2) 0.52
a
Secondary Endpoint Change in CGI-S (SE) -0.6 (0.06) -0.5 (0.06) -0.1 (-0.3, 0.04) 0.14
a
Post-Hoc Subgroup Analysis
Risperidone
Change in PANSS
Total Score (SE)
(N=60) (N=69)
1.1 (-3.7, 5.9) 0.66
a
-11.3 (2.13) -12.3 (2.10)
Non-Risperidone
Change in PANSS
Total Score (SE)
(N=130) (N=127)
-3.4 (-6.3, -0.5) 0.03
a
-15.1 (1.18) -11.7 (1.17)
F
O
R
P
E
R
S
O
N
A
L
U
S
E
O
N
L
Y](https://image.slidesharecdn.com/244schizpresentationforslideshare-251006132442-78e94853/75/Addressing-Positive-Negative-and-Cognitive-Symptoms-of-Schizophrenia-New-and-Emerging-Strategies-for-Success-26-2048.jpg)
![Emraclidine: M4-Positive Allosteric Modulator
In the EMPOWER phase 2b trials, emraclidine was well-tolerated with a safety profile comparable to that observed in
the phase 1b trial. The most commonly reported adverse events in EMPOWER-1 and EMPOWER-2, respectively, were:
Headache (9.4% and 10.8% in placebo;14.1% in EMPOWER-1 10 mg; 14.6% in EMPOWER-2 15 mg; 13.2% and 13.0% in 30
mg)
Dry mouth (2.3% and 0.8% in placebo; 3.9% in EMPOWER-1 10 mg; 0.8% in EMPOWER-2 15 mg; 9.3% and 5.3% in 30 mg)
Dyspepsia (3.1% and 1.5% in placebo; 3.9% in EMPOWER-1 10 mg; 3.1% in EMPOWER-2 15 mg; 7.8% and 2.3% in 30 mg)
QD = once daily.
Krystal et al (2022). Lancet, 400(10369):2210-2220;r AbbVie, 2024 [https://news.abbvie.com/2024-11-11-
AbbVie-Provides-Update-on-Phase-2-Results-for-Emraclidine-in-Schizophrenia].
Phase 2b EMPOWER Studies vs Phase 1b Study
EMPOWER-1 EMPOWER-2
Placebo
(n=127)
Emraclidine
10 mg QD (n=125)
Emraclidine
30 mg QD (n=127)
Placebo (n=128)
Emraclidine
15 mg QD (n=122)
Emraclidine
30 mg QD (n=123)
Baseline (SD) 98.3 (8.2) 97.6 (7.6) 97.9 (7.9) 97.4 (8.2) 98.0 (8.5) 97.2 (7.8)
LS mean (95% CI)
-13.5
(-17.0, -10.0)
-14.7
(-18.1, -11.2)
-16.5
(-20.0, -13.1)
-16.1
(-19.4, -12.8)
-18.5
(-22.0, -15.0)
-14.2
(-17.6, -10.8)
--------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
Phase 1b study
Placebo
(n=27)
Emraclidine
30 mg QD (n=27)
Emraclidine
20 mg BID (n=27)
Baseline (SD) 93 (8.8) 93 (7.3) 97 (7.9)
LS mean (SE) -6.8 (3.8) -19.5 (3.9) -17.9 (3.9)
F
O
R
P
E
R
S
O
N
A
L
U
S
E
O
N
L
Y](https://image.slidesharecdn.com/244schizpresentationforslideshare-251006132442-78e94853/75/Addressing-Positive-Negative-and-Cognitive-Symptoms-of-Schizophrenia-New-and-Emerging-Strategies-for-Success-27-2048.jpg)
![LB-102
GlobeNewswire, 2025 [https://www.globenewswire.com/news-release/2025/01/08/3006104/0/en/LB-Pharmaceuticals
-Announces-Positive-Topline-Results-from-Phase-2-Trial-of-LB-102-in-Schizophrenia.html].
NOVA1 met its primary endpoint, demonstrating statistically significant
change from baseline in the PANSS total score at 4 weeks
The 50-mg dose arm (n=107) achieved an effect size of 0.61, and
participants experienced a 5.0-point reduction in PANSS total score
compared to placebo (P=0.0009)
Treatment with the 75-mg dose (n=108) achieved an effect size of 0.41 and
led to a 4.7-point reduction in PANSS total score compared to placebo
(P=0.0022)
The study also included an exploratory dose of 100 mg (n=36), which
demonstrated an effect size of 0.83 with a 6.8-point reduction in PANSS total
score compared to placebo (P=0.0017)
Methylated Version of D2/3-5HT7 Antagonist Amisulpride
F
O
R
P
E
R
S
O
N
A
L
U
S
E
O
N
L
Y](https://image.slidesharecdn.com/244schizpresentationforslideshare-251006132442-78e94853/75/Addressing-Positive-Negative-and-Cognitive-Symptoms-of-Schizophrenia-New-and-Emerging-Strategies-for-Success-30-2048.jpg)
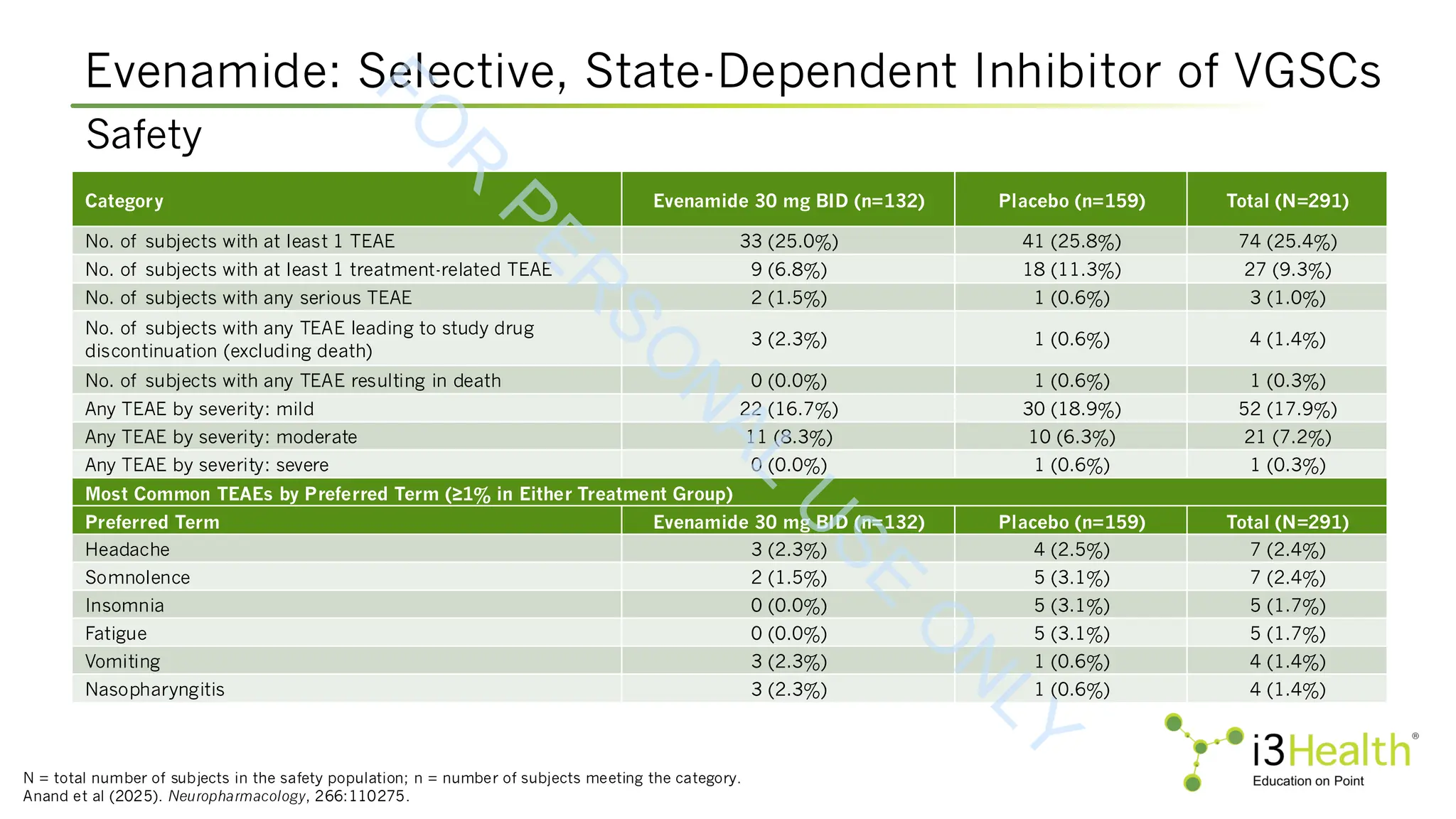
![aResponder rates were calculated as number of observed patients meeting responder criterion divided by the total N under mITT
population in each treatment arm.
bP value from logistic regression model (chi-square) comparing proportion of responders in evenamide 30 mg BID vs placebo BID.
CGI-C = Clinical Global Impression – Corrections.
Anand et al (2025). Neuropharmacology, 266:110275.
Selective, State-Dependent Inhibitor of Voltage-Gated Sodium Channels (VGSCs)
Evenamide for Positive Symptom Partial Non-Responders
Analysis Population
Responder
Category
Responders
[n/N (%)]
Odds Ratio
(95% CI)
P Value
b
mITT populationa
PANSS ≥20%
Evenamide
27/131 (20.6%)
1.99 (1.0, 3.8) 0.037
Placebo
18/156 (11.5%)
CGI-C ≤2
Evenamide
41/131 (31.3%)
2.18 (1.2, 3.8) 0.006
Placebo
27/156 (17.3%)
mITT population:
observed cases
PANSS ≥20%
Evenamide
27/127 (21.3%)
2.04 (1.1, 3.9) 0.032
Placebo
18/154 (11.7%)
CGI-C ≤2
Evenamide
41/127 (32.3%)
2.24 (1.3, 3.9) 0.005
Placebo
27/154 (17.5%)
F
O
R
P
E
R
S
O
N
A
L
U
S
E
O
N
L
Y](https://image.slidesharecdn.com/244schizpresentationforslideshare-251006132442-78e94853/75/Addressing-Positive-Negative-and-Cognitive-Symptoms-of-Schizophrenia-New-and-Emerging-Strategies-for-Success-31-2048.jpg)

![Olanzapine: Long-Acting Subcutaneous Antipsychotic (TV-44749)
Shulman et al (2024). [Poster presentation] Psych Congress 2024. Poster 96.
Efficacy in the Acute 8-Week Phase 1 of the SOLARIS Trial
Superiority of TV-44749 vs
placebo on all secondary
outcomes; no postinjection
somnolence sedation
(PDSS) events in
>3,300 injections
SOLARIS phase 2 open
extension study ongoing
Figure 3: LS mean change from baseline in the PANSS total score
F
O
R
P
E
R
S
O
N
A
L
U
S
E
O
N
L
Y](https://image.slidesharecdn.com/244schizpresentationforslideshare-251006132442-78e94853/75/Addressing-Positive-Negative-and-Cognitive-Symptoms-of-Schizophrenia-New-and-Emerging-Strategies-for-Success-40-2048.jpg)
![Olanzapine: Safety and Tolerability
Shulman et al (2024). [Poster presentation] Psych Congress 2024. Poster 96.
SOLARIS: Phase 1 Acute 8-Week
Parameter, n (%) Placebo
(n=167)
TV-44749
318 mg
(n=163)
TV-44749
425 mg
(n=168)
TV-44749
531 mg
(n=169)
TV-44749
Total
(N=500)
≥1 adverse event 84 (50%) 112 (69%) 117 (70%) 126 (75%) 355 (71%)
≥1 serious adverse event 3 (2%) 4 (2%) 1 (<1%) 2 (1%) 7 (1%)
≥1 severe adverse event 4 (2%) 5 (3%) 1 (<1%) 3 (2%) 9 (2%)
Overall discontinuation rates 49 (29%) 54 (32%) 48 (28%) 48 (28%) 150 (30%)
≥1 adverse event leading to stopping treatment 5 (3%) 7 (4%) 4 (2%) 5 (3%) 16 (3%)
≥1 treatment-related adverse event 40 (24%) 85 (52%) 95 (57%) 103 (61%) 283 (57%)
≥1 adverse event leading to stopping the study 6 (4%) 8 (5%) 7 (4%) 6 (4%) 21 (4%)
Adverse events in >5% of patients and being higher than in the placebo group
Weight increased 13 (8%) 49 (30%) 66 (39%) 58 (34%) 173 (35%)
Injection site induration 4 (2%) 18 (11%) 23 (14%) 23 (14%) 64 (13%)
Injection site pain 7 (4%) 14 (9%) 19 (11%) 17 (10%) 50 (10%)
Injection site erythema 1 (<1%) 12 (7%) 21 (13%) 15 (9%) 48 (10%)
Somnolence 3 (2%) 16 (10%) 10 (6%) 13 (8%) 39 (8%)
F
O
R
P
E
R
S
O
N
A
L
U
S
E
O
N
L
Y](https://image.slidesharecdn.com/244schizpresentationforslideshare-251006132442-78e94853/75/Addressing-Positive-Negative-and-Cognitive-Symptoms-of-Schizophrenia-New-and-Emerging-Strategies-for-Success-41-2048.jpg)
![Potential for TV-44749 to Eliminate PDSS Risk
aSimulated data using a population PK model that was constructed based on actual data from phase 1 trial.
PK = pharmacokinetic.
Krtalić et al (2024). [Poster presentation] Schizophrenia International Research Society 2024 Annual Congress. Poster S82;
Perlstein et al (2024). [Oral presentation] Schizophrenia International Research Society 2024 Annual Congress.
Median
(5
th
-95
th
)
concentration
(ng/mL)
Time (days)
0
20
40
60
80
100
120
0 4 8 12 16 20 24 28
Once-monthly subcutaneous TV-44749 425 mg
Oral olanzapine 15 mg/day
Controlled release
of olanzapine shown
by in vitro data
In first-in-humans phase 1 study, clinically relevant therapeutic olanzapine plasma concentrations were
attained within 1-2 days (without a loading dose or complex initiation regimen) and maintained during
the 28-day dosing interval with no confirmed or suspected cases of PDSS
Sustained release of
olanzapine shown
by simulateda
population PK data
In vitro and early clinical data show the potential for TV-44749 to
eliminate postinjection delirium/sedation syndrome (PDSS) risk
F
O
R
P
E
R
S
O
N
A
L
U
S
E
O
N
L
Y](https://image.slidesharecdn.com/244schizpresentationforslideshare-251006132442-78e94853/75/Addressing-Positive-Negative-and-Cognitive-Symptoms-of-Schizophrenia-New-and-Emerging-Strategies-for-Success-42-2048.jpg)


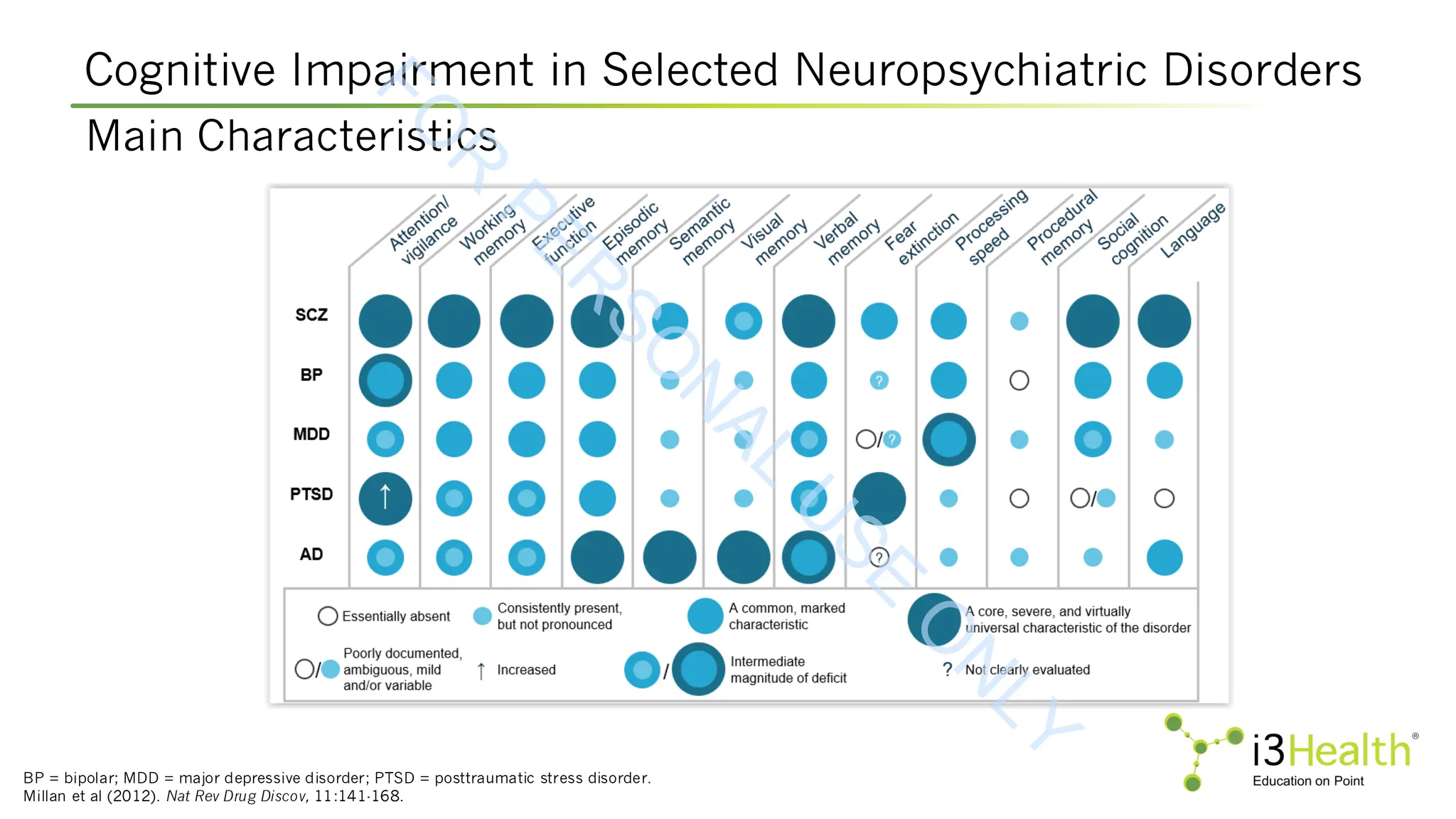
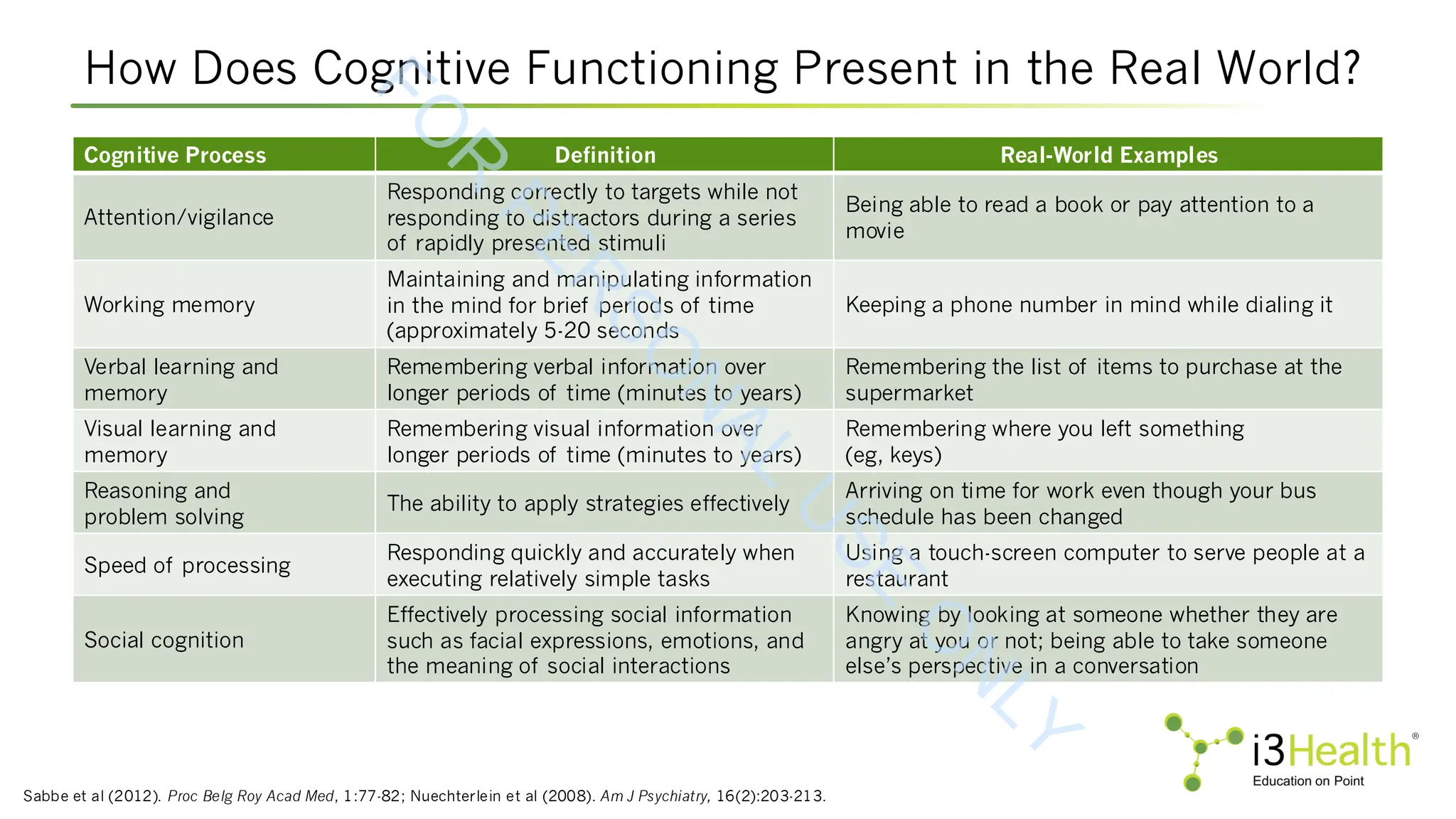
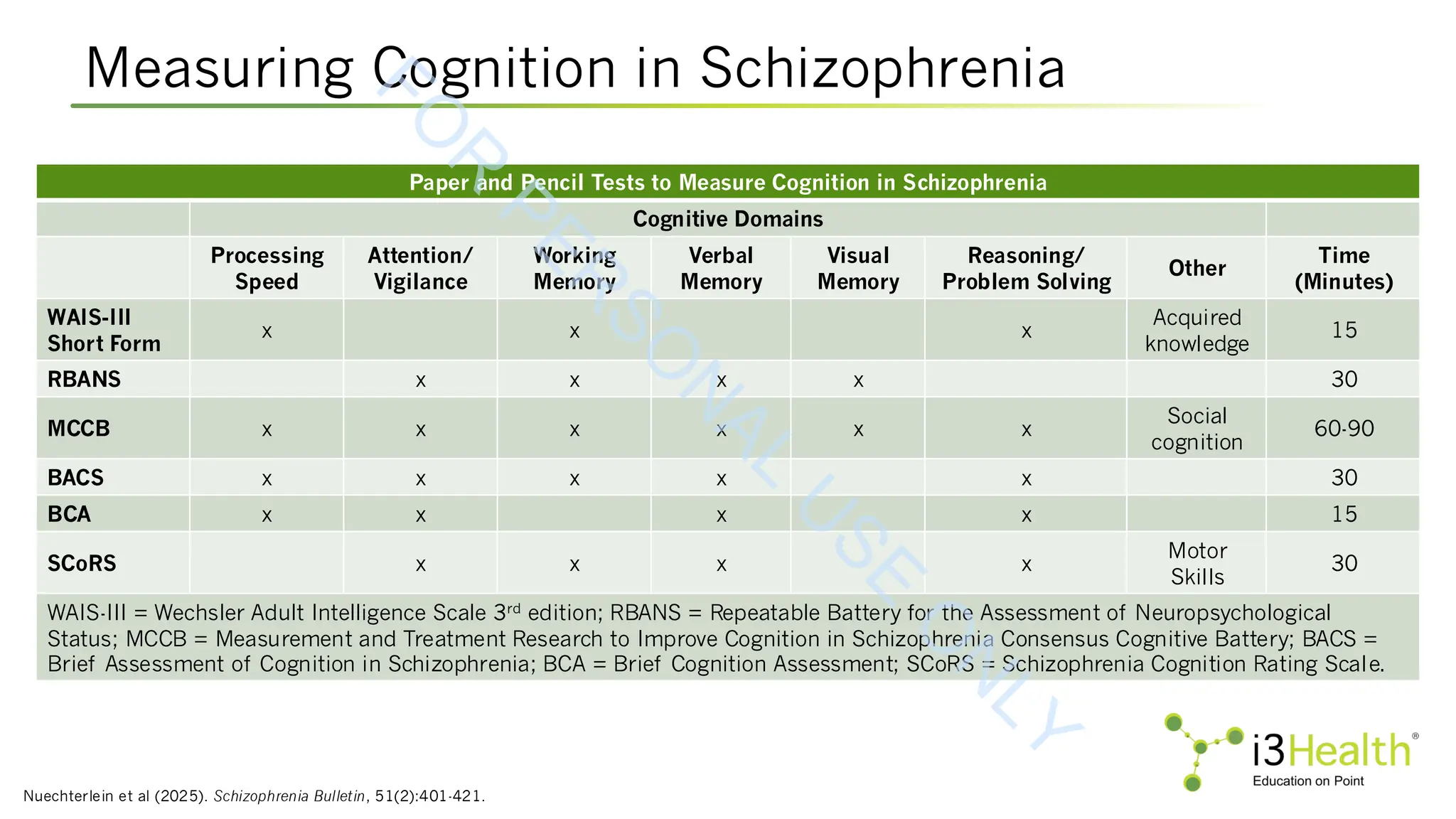
![Pharmacotherapies in Development for CIAS
MOA = mechanism of action; CIAS = cognitive impairment associated with schizophrenia; ASCP = American Society for Clinical Pathology.
NCT05686239; NCT04972227; NCT04457310;
Harvey et al (2023). [Poster presentation]. American Society of Clinical Psychopharmacology Annual Meeting, 2023. Abstract W77.
Therapy Class/MOA Indication Status Clinical Trial
RL-007
GABA-B receptor agonist, NMDA receptor
agonist, nicotinic receptors agonist
CIAS Phase 2 NCT05686239
CY6463
CNS-penetrant soluble guanylate cyclase
(sGC) stimulator, a key enzyme of the nitric
oxide (NO) signaling pathway
CIAS Phase 1 NCT04972227
CVL-562 Dopamine 1 partial agonist CIAS Phase 1/2 NCT04457310
KarXT M1/M4 muscarinic agonist
Schizophrenia (cognitive data
obtained in acute trials)
Phase 2/3
Harvey et al,
ASCP 2023
F
O
R
P
E
R
S
O
N
A
L
U
S
E
O
N
L
Y](https://image.slidesharecdn.com/244schizpresentationforslideshare-251006132442-78e94853/75/Addressing-Positive-Negative-and-Cognitive-Symptoms-of-Schizophrenia-New-and-Emerging-Strategies-for-Success-56-2048.jpg)
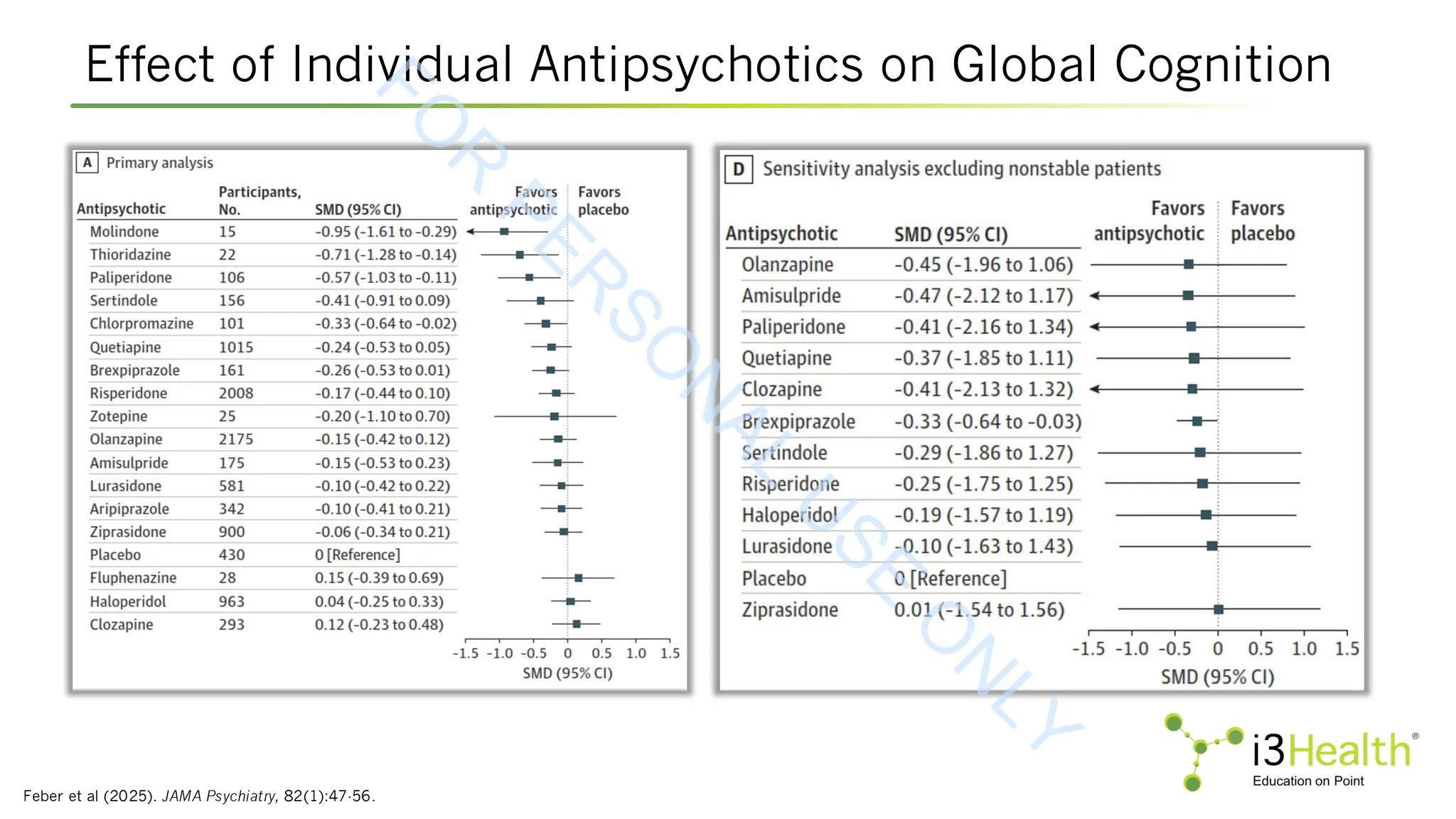
![KarXT EMERGENT-1, EMERGENT-2, EMERGENT-3
CANTAB = Cambridge Neuropsychological Test Automated Battery.
Harvey et al (2023). [Poster presentation]. American Society of Clinical Psychopharmacology Annual Meeting, 2023. Abstract W77.
Change in Cognition From Baseline to Week 5
F
O
R
P
E
R
S
O
N
A
L
U
S
E
O
N
L
Y](https://image.slidesharecdn.com/244schizpresentationforslideshare-251006132442-78e94853/75/Addressing-Positive-Negative-and-Cognitive-Symptoms-of-Schizophrenia-New-and-Emerging-Strategies-for-Success-57-2048.jpg)








![References (cont.)
Feber L, Peter NL, Chiocchia V, et al (2025). Antipsychotic drugs and cognitive function: a systematic review and network meta-analysis. JAMA Psychiatry, 82(1):47-56.
DOI:10.1001/jamapsychiatry.2024.2890
GlobeNewswire.com (2025). LB pharmaceuticals announces positive topline results from phase 2 trial of LB-102 in schizophrenia. Available at:
https://www.globenewswire.com/news-release/2025/01/08/3006104/0/en/LB-Pharmaceuticals-Announces-Positive-Topline-Results-from-Phase-2-Trial-of-LB-102-in-
Schizophrenia.html
Goff DC, Hill M & Freudenreich O (2010). Strategies for improving treatment adherence in schizophrenia and schizoaffective disorder. J Clin Psychiatry, 71(suppl_2):20-26.
DOI:10.4088/JCP.9096su1cc.04.
Grunder G, Carlsson A & Wong DF (2003). Mechanism of new antipsychotic medications: occupancy is not just antagonism. Arch Gen Psychiatry, 60(10):974-977.
DOI:10.1001/archpsyc.60.10.974
Haddad PM, Brain C & Scott J (2014) Nonadherence with antipsychotic medication in schizophrenia: challenges and management strategies. Patient Relat Outcome Meas, 5:43-62.
DOI:10.2147/PROM.S42735
Harvey PD, Saunder C, Yohn SE et al (2023). The potential role of the M1/M4 muscarinic receptor agonist KarTX in the treatment of cognitive impairment in patients with
schizophrenia. [Poster presentation]. American Society of Clinical Psychopharmacology Annual Meeting, 2023. Abstract W77.
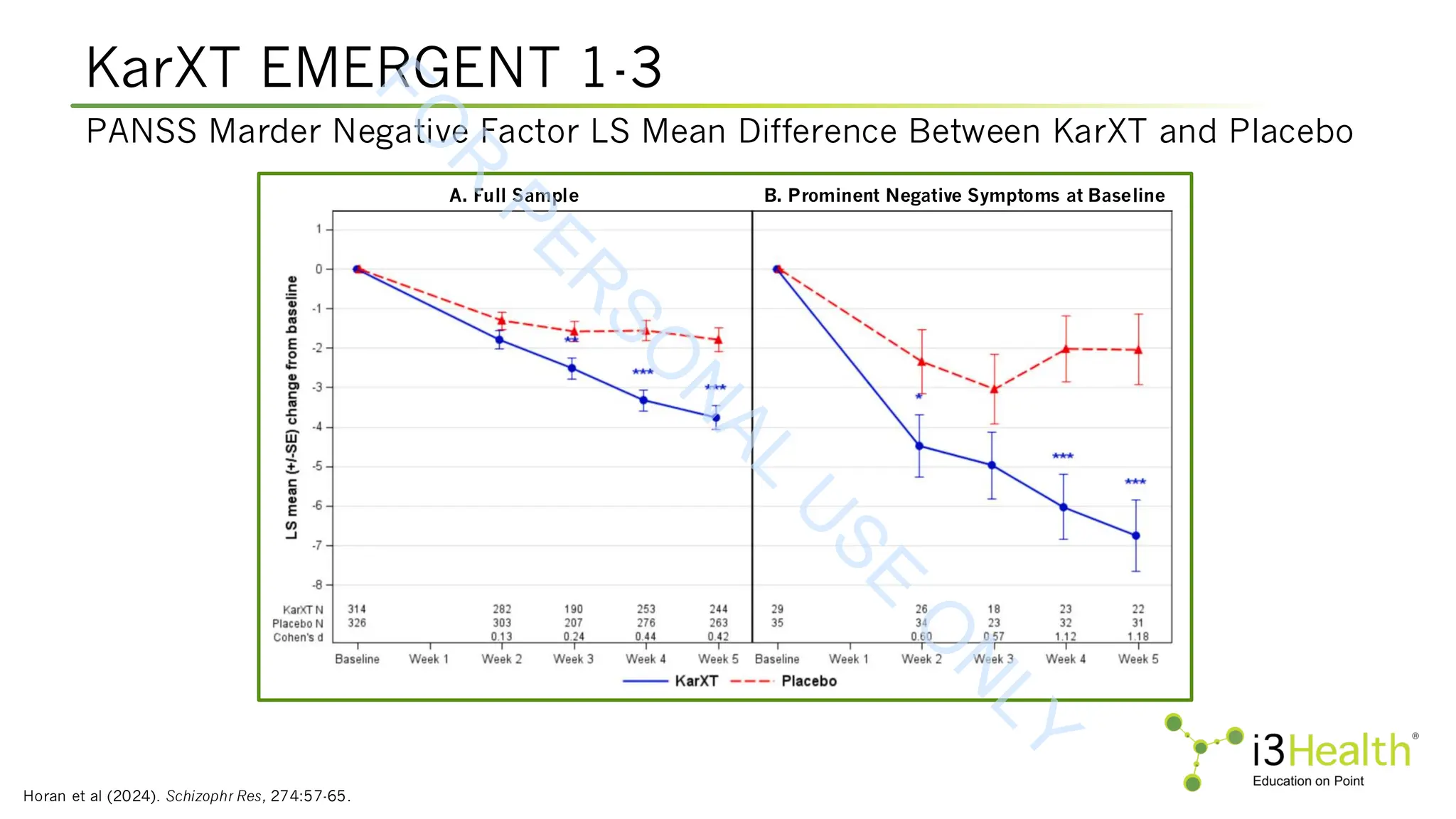
Horan WP, Targum SD, Claxton A, et al (2024). Efficacy of KarXT on negative symptoms in acute schizophrenia: a post hoc analysis of pooled data from 3 trials. Schizophr Res,
274:57-65. DOI:10.1016/j.schres.2024.08.001
Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al (2019). Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode
schizophrenia: a systematic review and network meta-analysis. Lancet, 394(10202):939-951. DOI:10.1016/S0140-6736(19)31135-3
Jääskeläinen E, Juola P, Hirvonen N, et al (2013). A systematic review and meta-analysis of recovery in schizophrenia. Schizophr Bull, 39(6):1296-1306.
DOI:10.1093/schbul/sbs130
Kadakia A, Catillon M, Fan Q, et al (2022). The economic burden of schizophrenia in the United States. J Clin Psychiatry, 83(6):22m14458. DOI:10.4088/JCP.22m14458
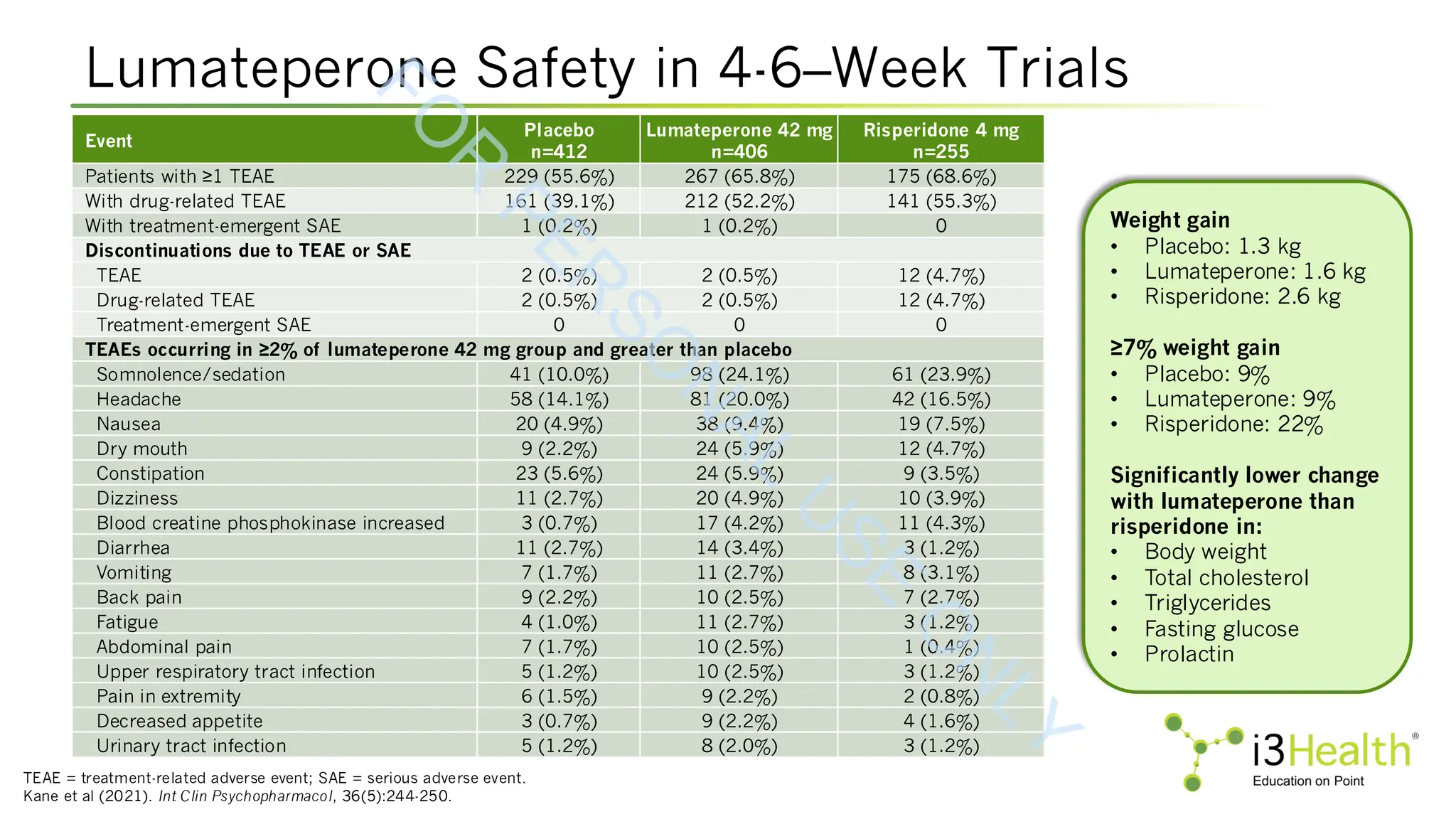
Kane JM, Durgam S, Satlin A, et al (2021). Safety and tolerability of lumateperone for the treatment of schizophrenia: a pooled analysis of late-phase placebo- and active-
controlled clinical trials. Int Clin Psychopharmacol, 36(5):244-250. DOI:10.1097/YIC.0000000000000371
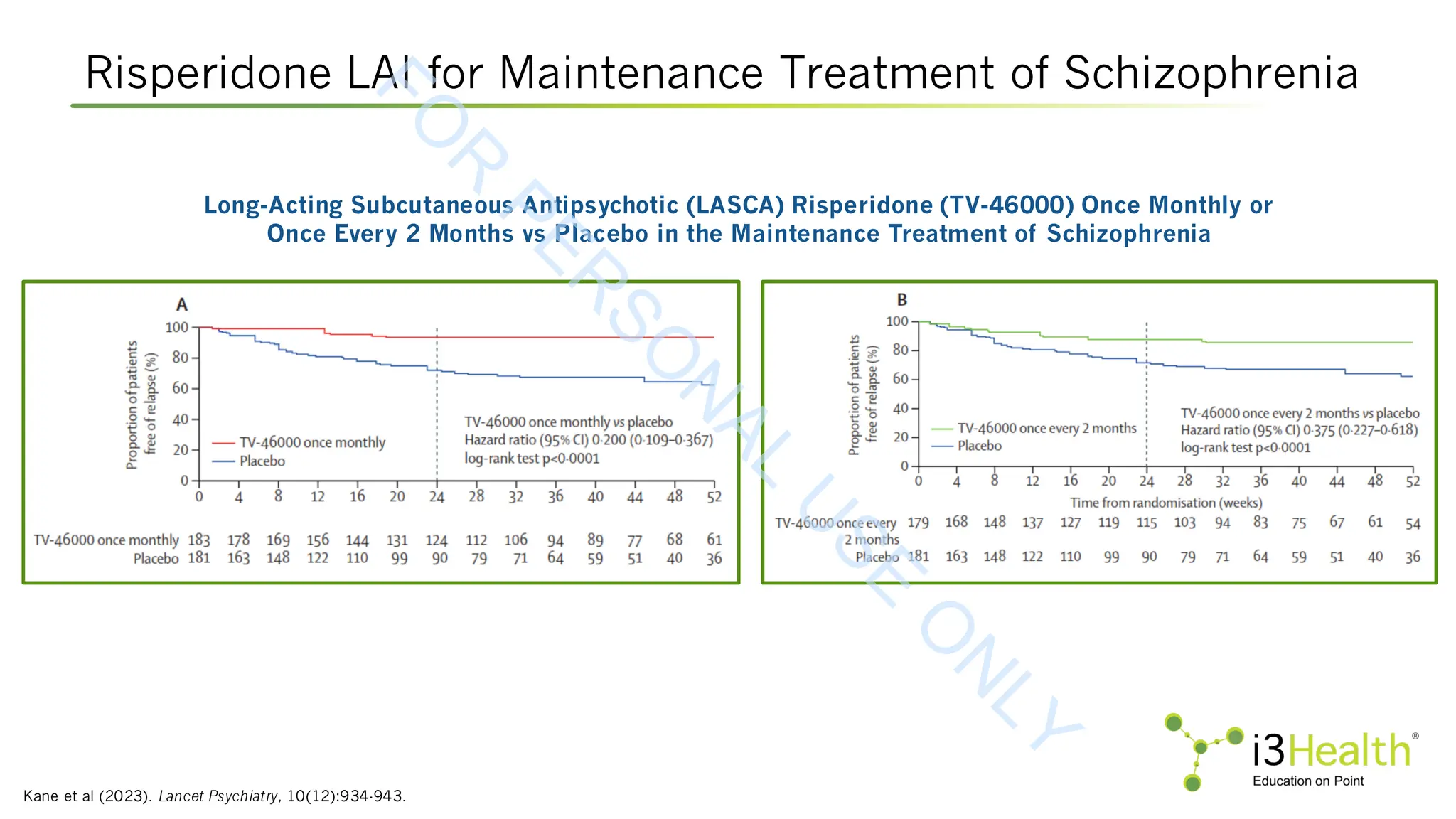
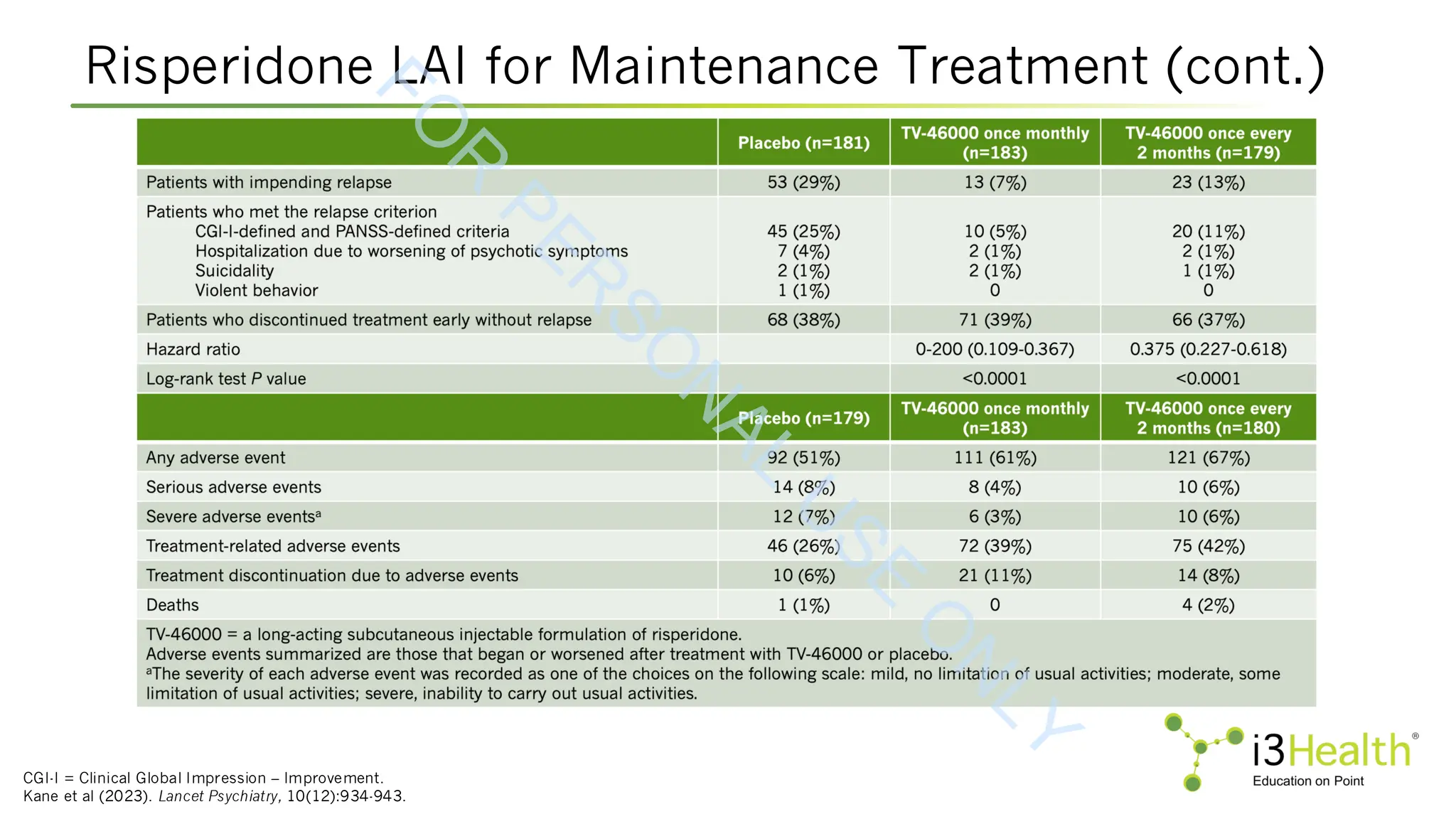
Kane JM, Harary E, Eshet R, et al (2023). Efficacy and safety of TV-46000, a long-acting, subcutaneous, injectable formulation of risperidone, for schizophrenia: a randomised
clinical trial in the USA and Bulgaria. Lancet Psychiatry, 10(12):934-943.
F
O
R
P
E
R
S
O
N
A
L
U
S
E
O
N
L
Y](https://image.slidesharecdn.com/244schizpresentationforslideshare-251006132442-78e94853/75/Addressing-Positive-Negative-and-Cognitive-Symptoms-of-Schizophrenia-New-and-Emerging-Strategies-for-Success-76-2048.jpg)
![References (cont.)
Kaul I, Sawchak S, Claxton A, et al (2024). Efficacy of xanomeline and trospium chloride in schizophrenia: pooled results from three 5-week, randomized, double-blind, placebo-
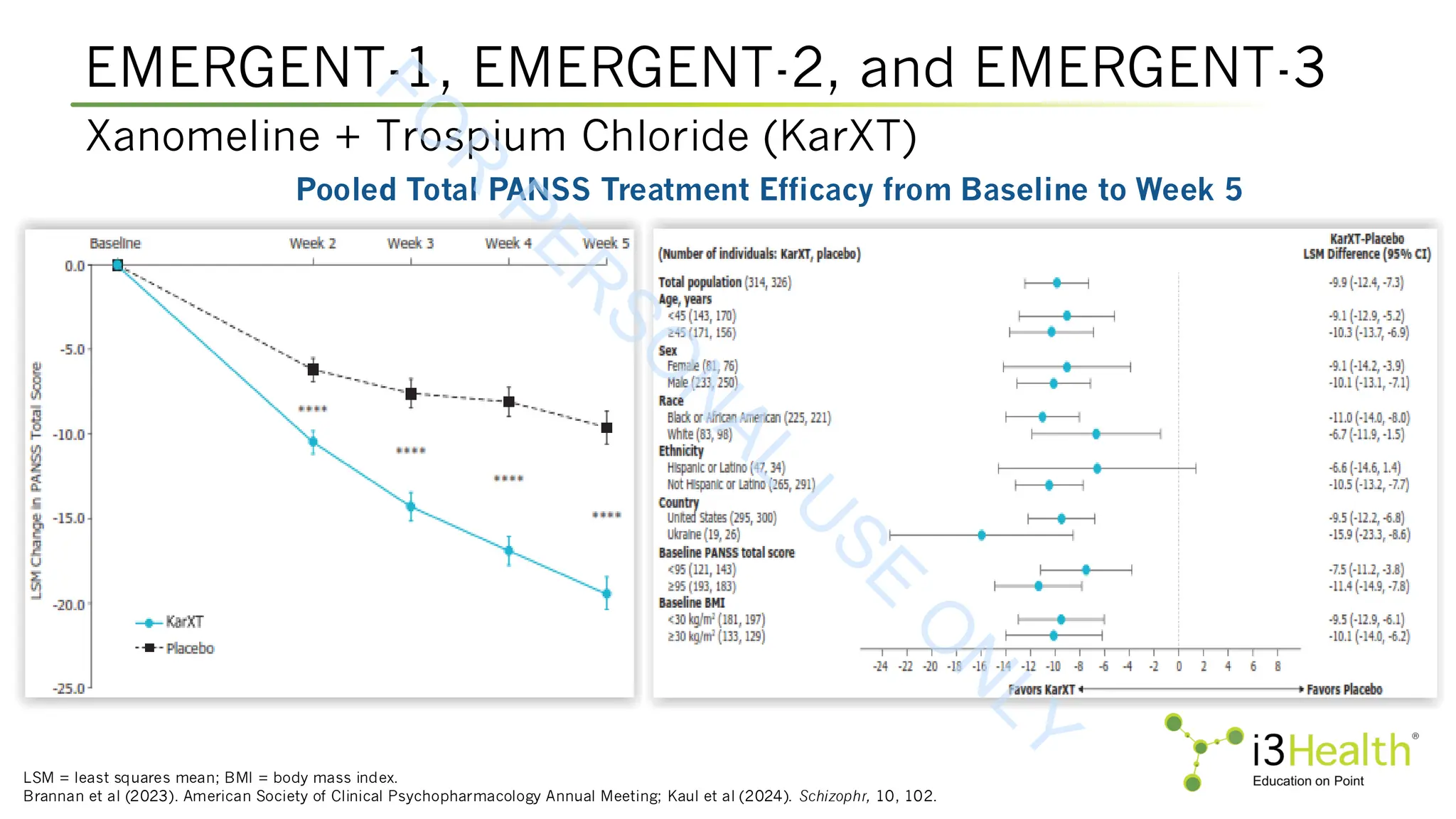
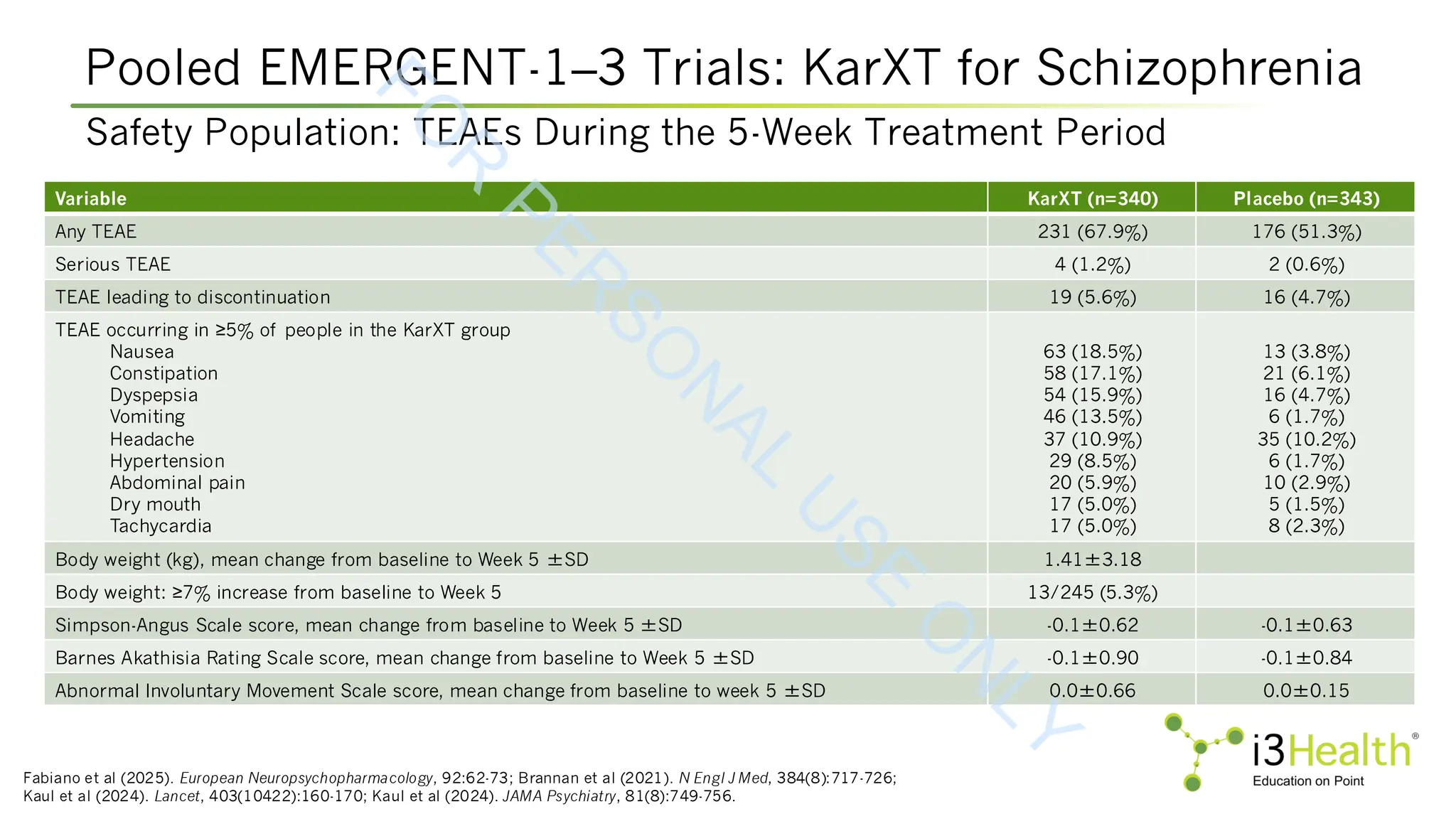
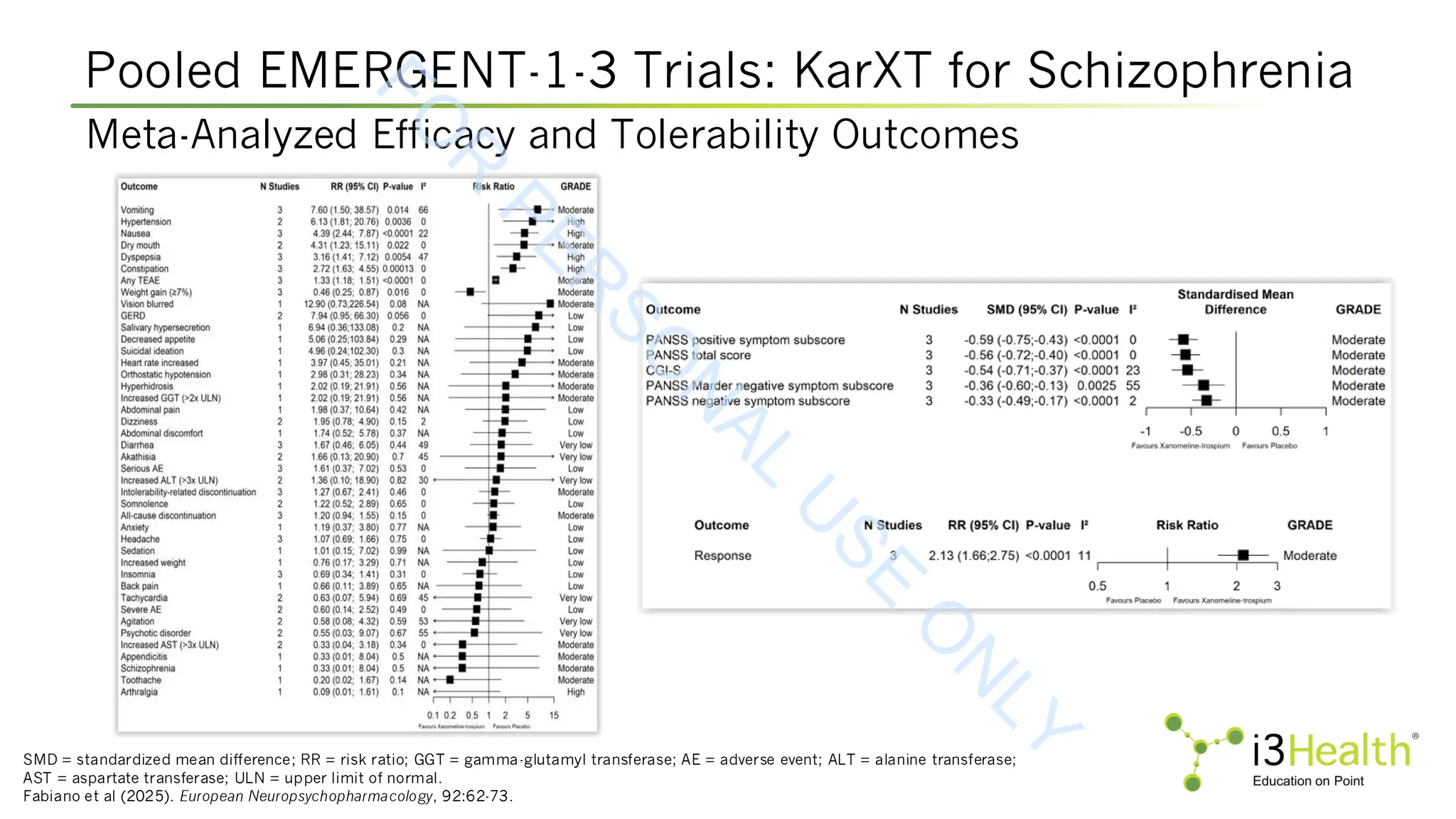
controlled, EMERGENT trials. Schizophr, 10, 102. DOI:10.1038/s41537-024-00525-6
Kaul I, Sawchak S, Correll CU, et al (2024). Efficacy and safety of the muscarinic receptor agonist KarXT (xanomeline-trospium) in schizophrenia (EMERGENT-2) in the USA:
results from a randomised, double-blind, placebo-controlled, flexible-dose phase 3 trial. Lancet, 403(10422):160-170. DOI:10.1016/S0140-6736(23)02190-6
Kaul I, Sawchak S, Walling DP, et al (2024). Efficacy and safety of xanomeline-trospium chloride in schizophrenia: a randomized clinical trial. JAMA Psychiatry. 81(8):749-756.
DOI:10.1001/jamapsychiatry.2024.0785
Keepers GA, Fochtmann LJ, Anzia JM (2020). The American Psychiatric Association practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry,
177(9):868-872. DOI:10.1176/appi.ajp.2020.177901.
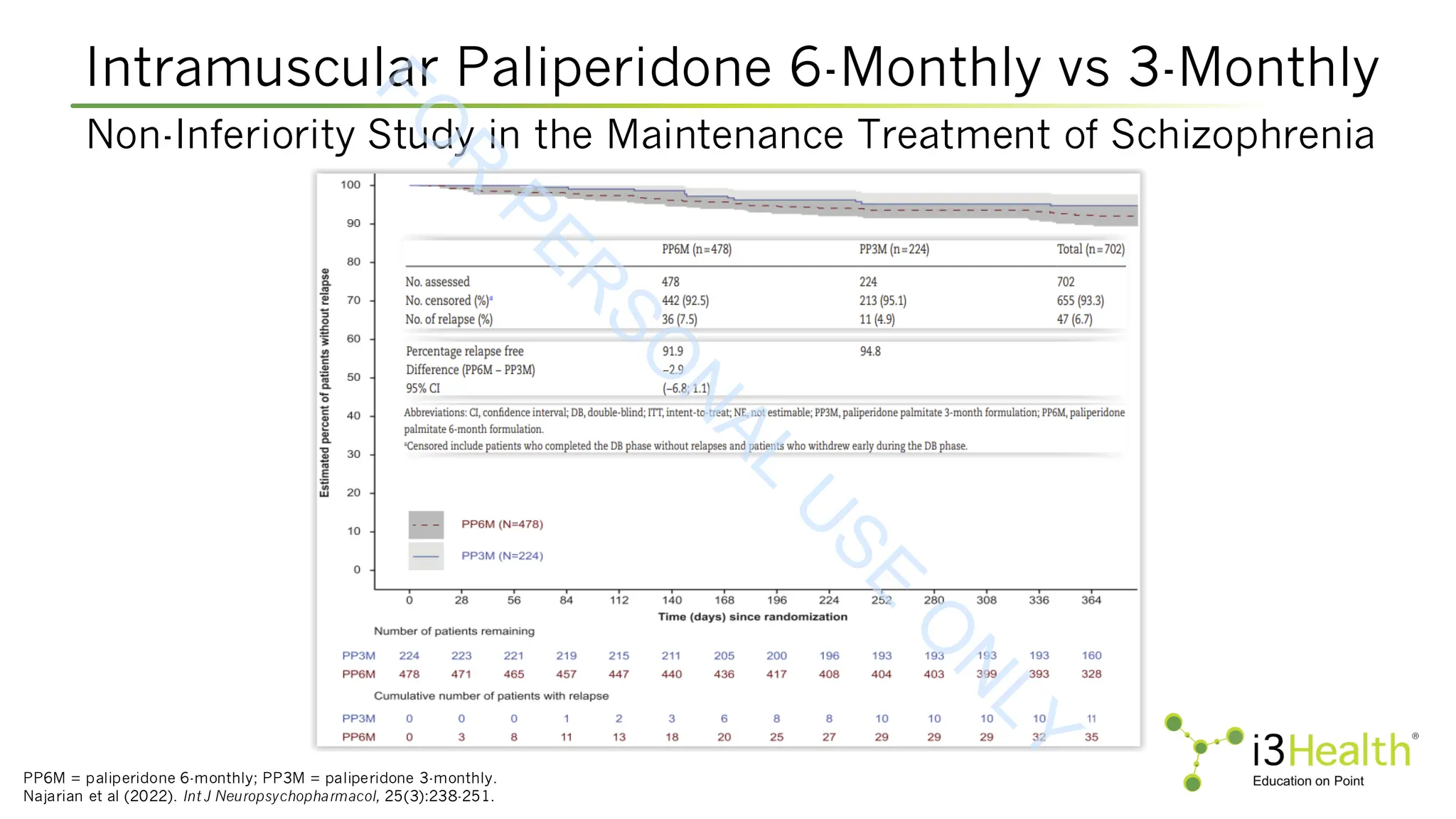
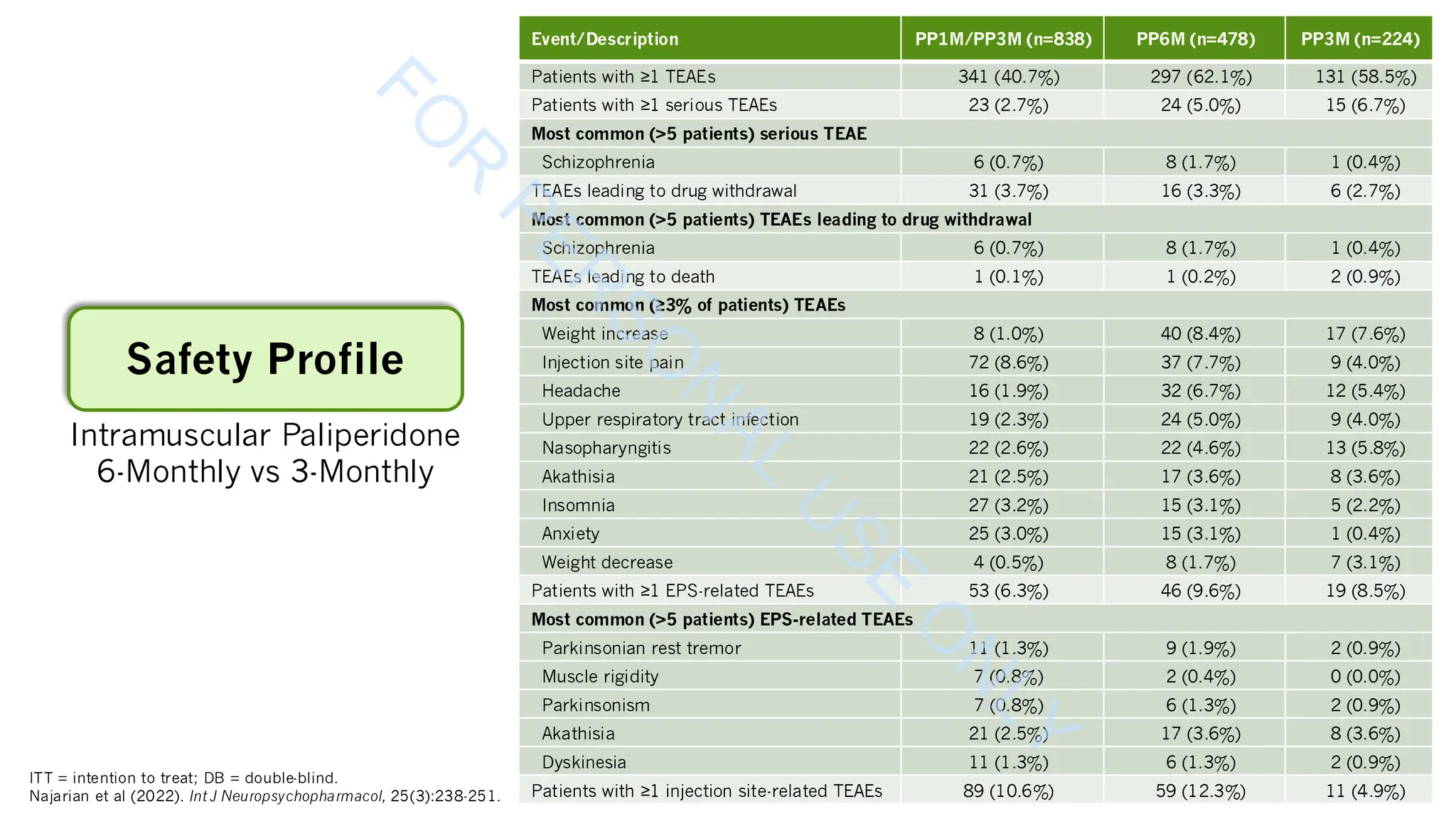
Kim E, Kim S, Kim SW, et al (2025). Stability of psychotic symptoms and safety in switching to aripiparazole once-monthly according to prior oral antipsychotic drugs. Schizophr
Res, 281:180-190. DOI:10.1016/j.schres.2025.04.033
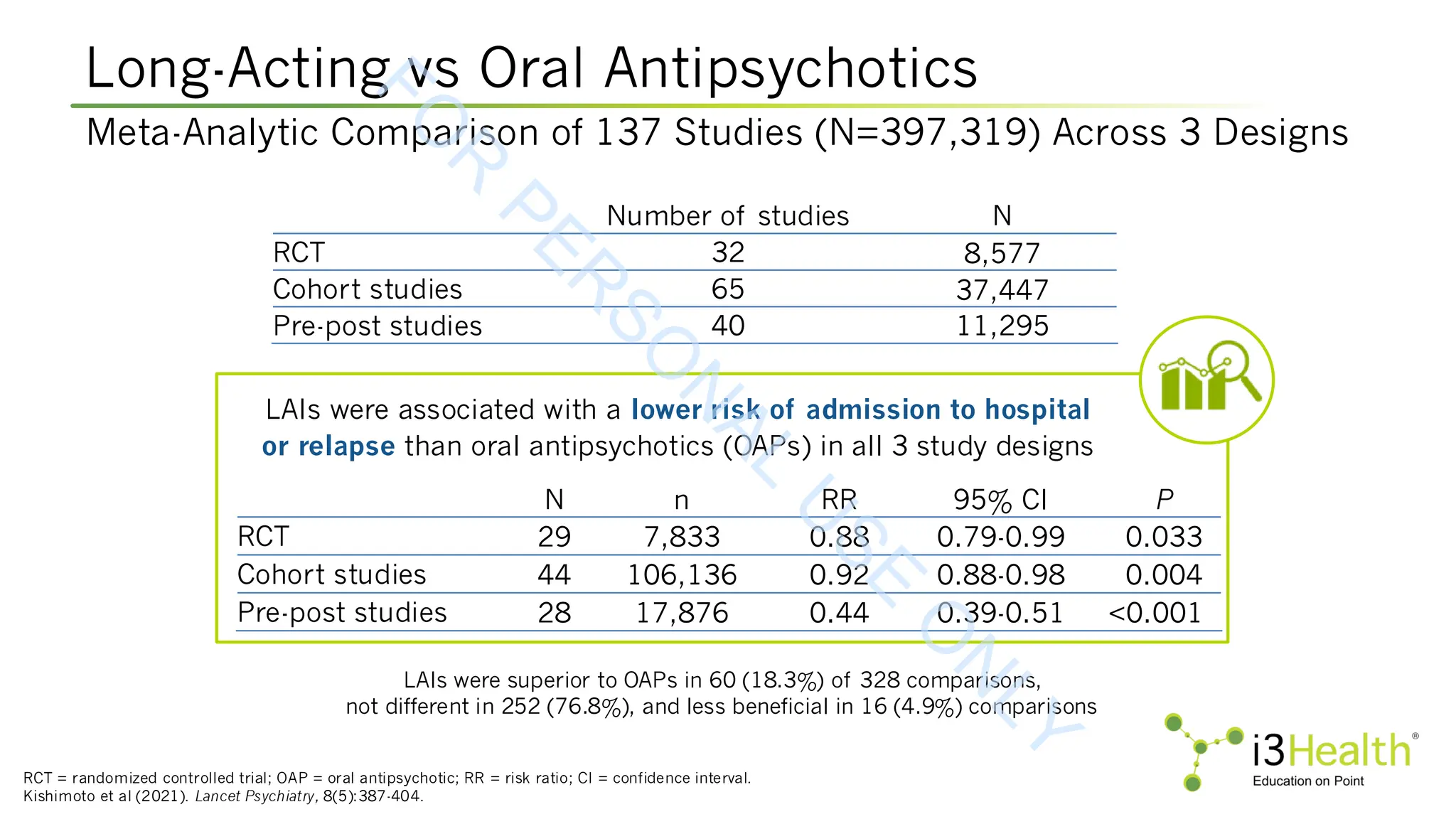
Kishimoto T, Hagi K, Kurokawa S, et al (2021). Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and
comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry, 8(5):387-404. DOI:10.1016/S2215-0366(21)00039-0
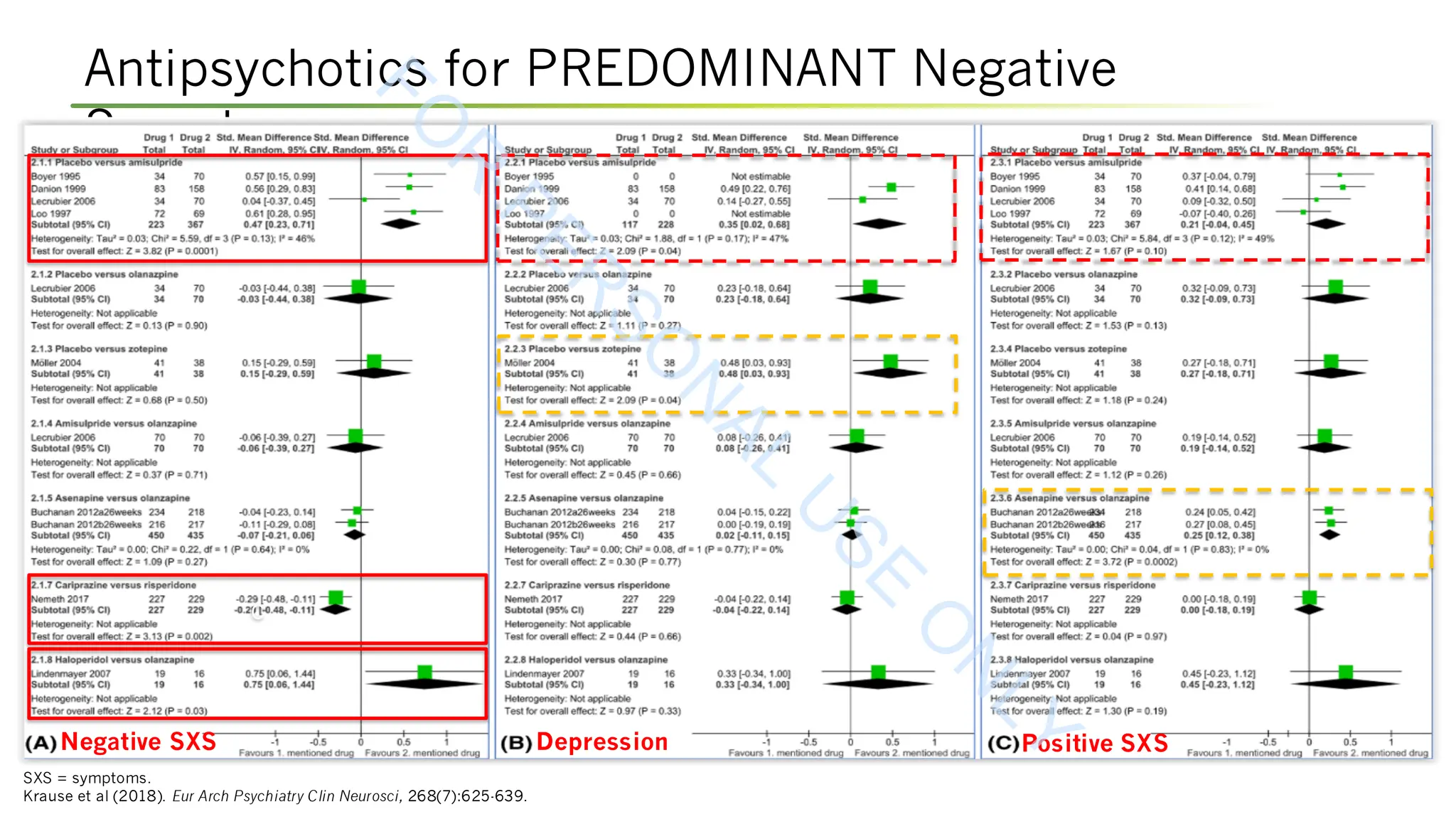
Krause M, Zhu Y, Huhn M, et al (2018). Antipsychotic drugs for patients with schizophrenia and predominant or prominent negative symptoms: a systematic review and meta-
analysis. Eur Arch Psychiatry Clin Neurosci, 268(7):625-639. DOI:10.1007/s00406-018-0869-3
Krtalić I, Juretić M, Komlosi A, et al (2024). TV-44749, a long-acting subcutaneous (SC) injectable formulation of olanzapine is designed to provide sustained controlled
concentrations and to elimitate the causes of post-injection delirium/sedation syndrome (PDSS). [Poster presentation]. Presented at the Schizophrenia International
Research Society 2024 Annual Congress. Abstract S82.
Krystal JH, Kane JM, Correll CU, et al (2022). Emraclidine, a novel positive allosteric modulator of cholinergic M4 receptors, for the treatment of schizophrenia: a two-part,
randomized, double-blind, placebo-controlled, phase 1b trial. Lancet, 400(10369):2210-2220. DOI:10.1016/S0140-6736(22)01990-0
Lieberman JA, Davis RE, Correll CU, et al (2016). ITI-007 for the treatment of schizophrenia: a 4-week randomized, double-blind, controlled trial, Biol Psychiatry, 79(12):952-961.
DOI:10.1016/j.biopsych.2015.08.026
Littrell KH & Littrell SH (1998). Issues of reintegration and rehabilitation in schizophrenia. Psychiatric Annals, 28(7):371-377. DOI:10.3928/0048-5713-19980701-07
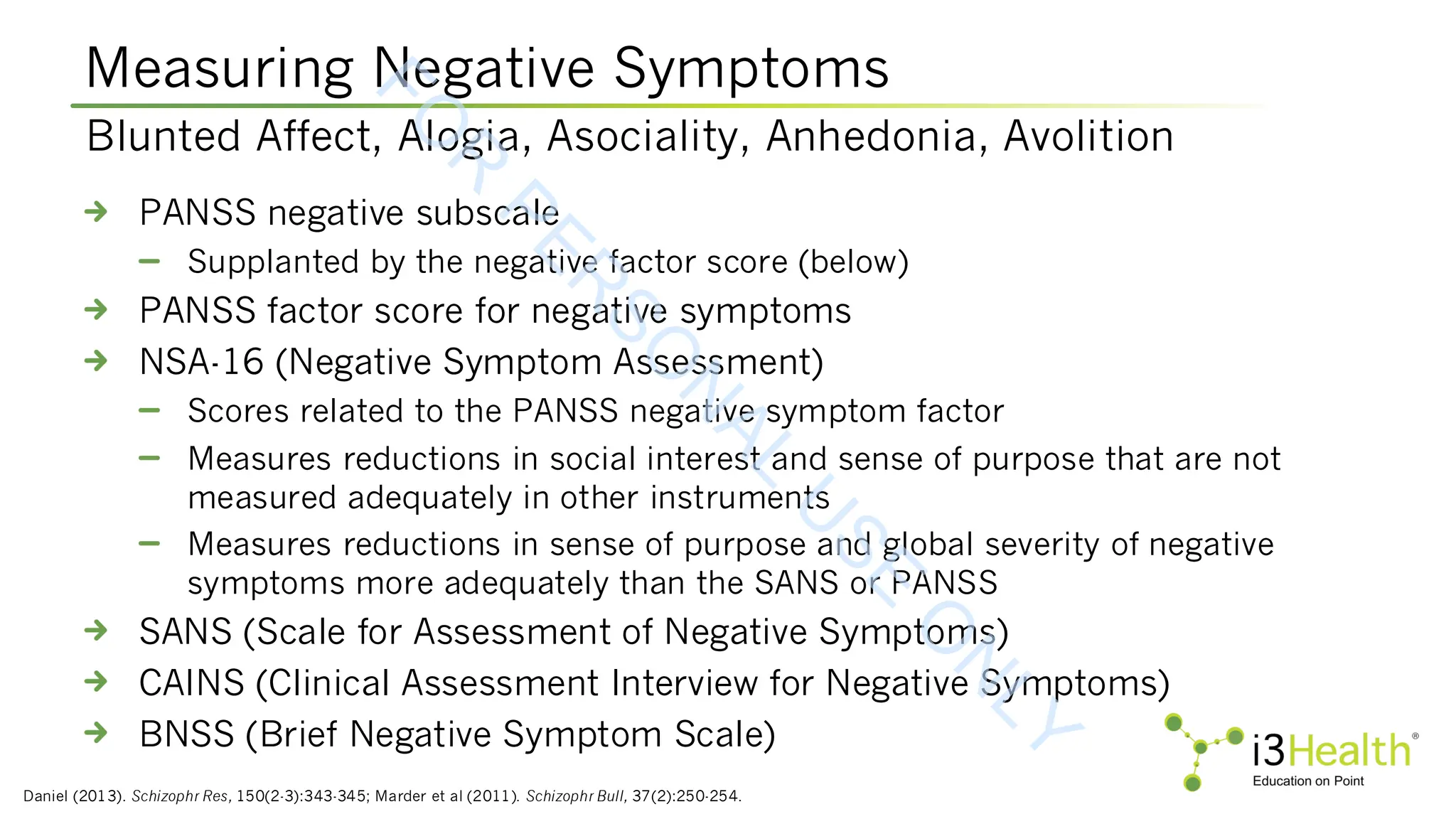
Marder SR, Daniel DG, Alphs L, et al (2011). Methodological issues in negative symptom trials. Schizophrenia Bulletin, 37(2):250-254. DOI:10.1093/schbul/sbq161
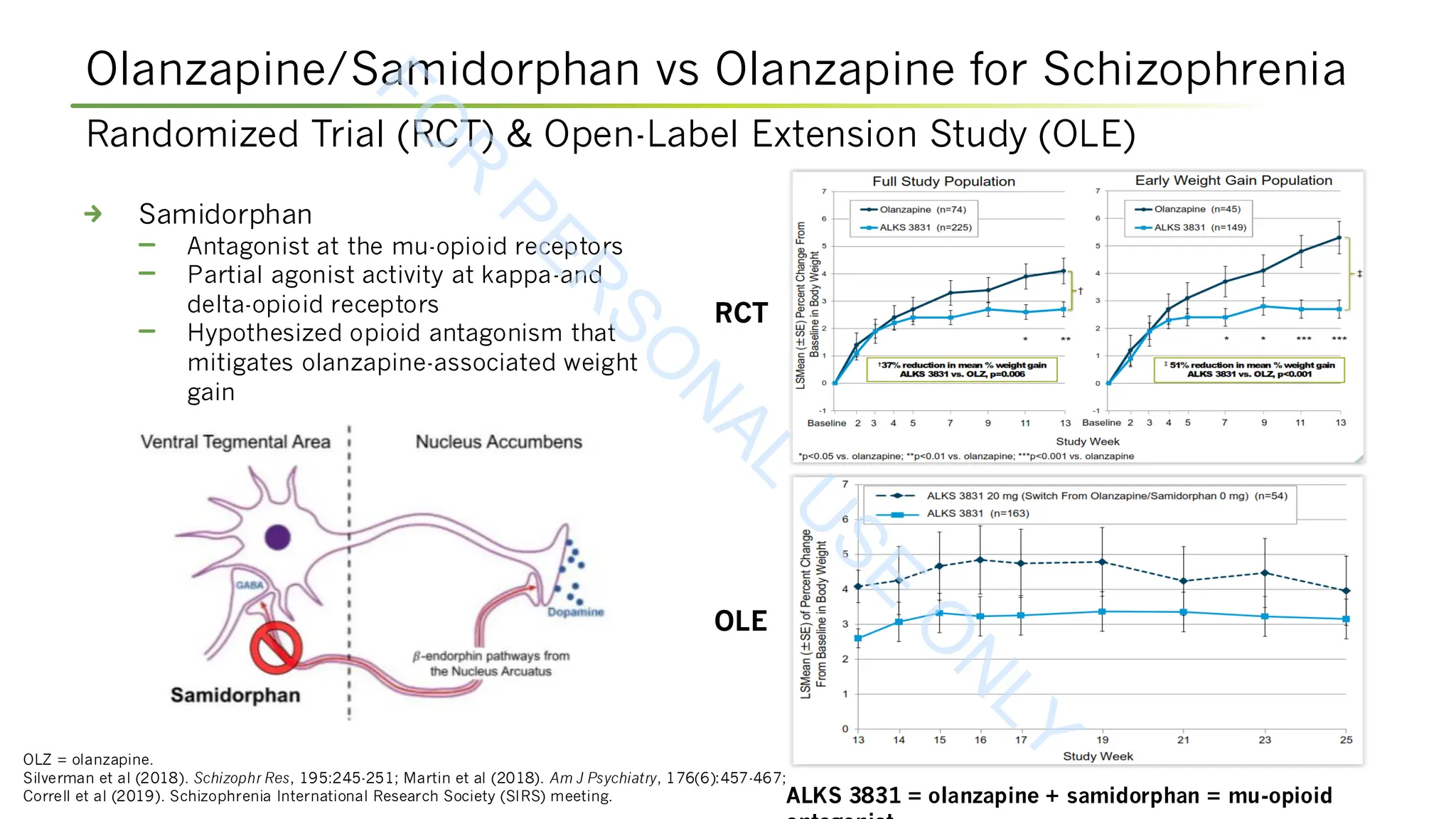
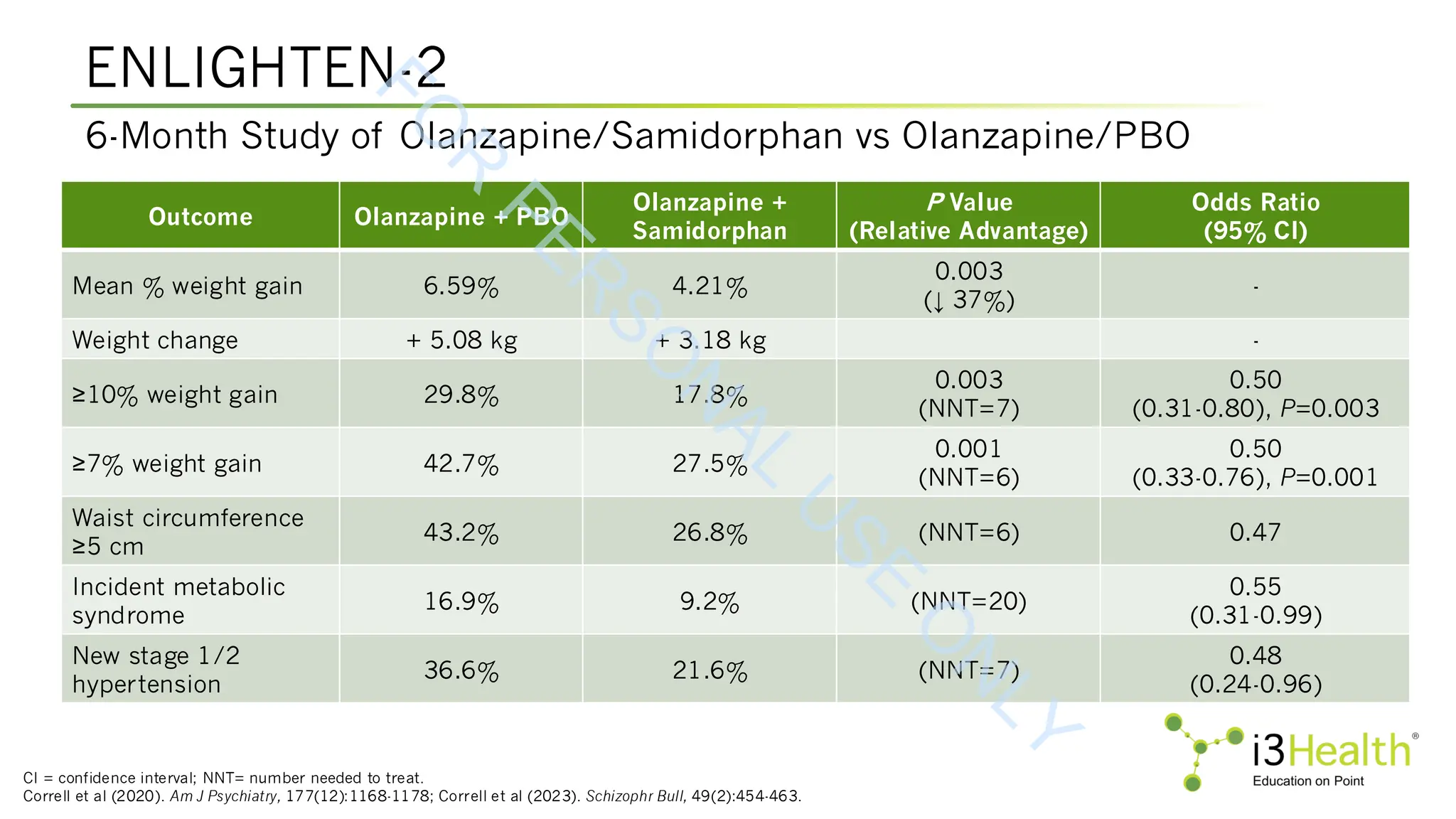
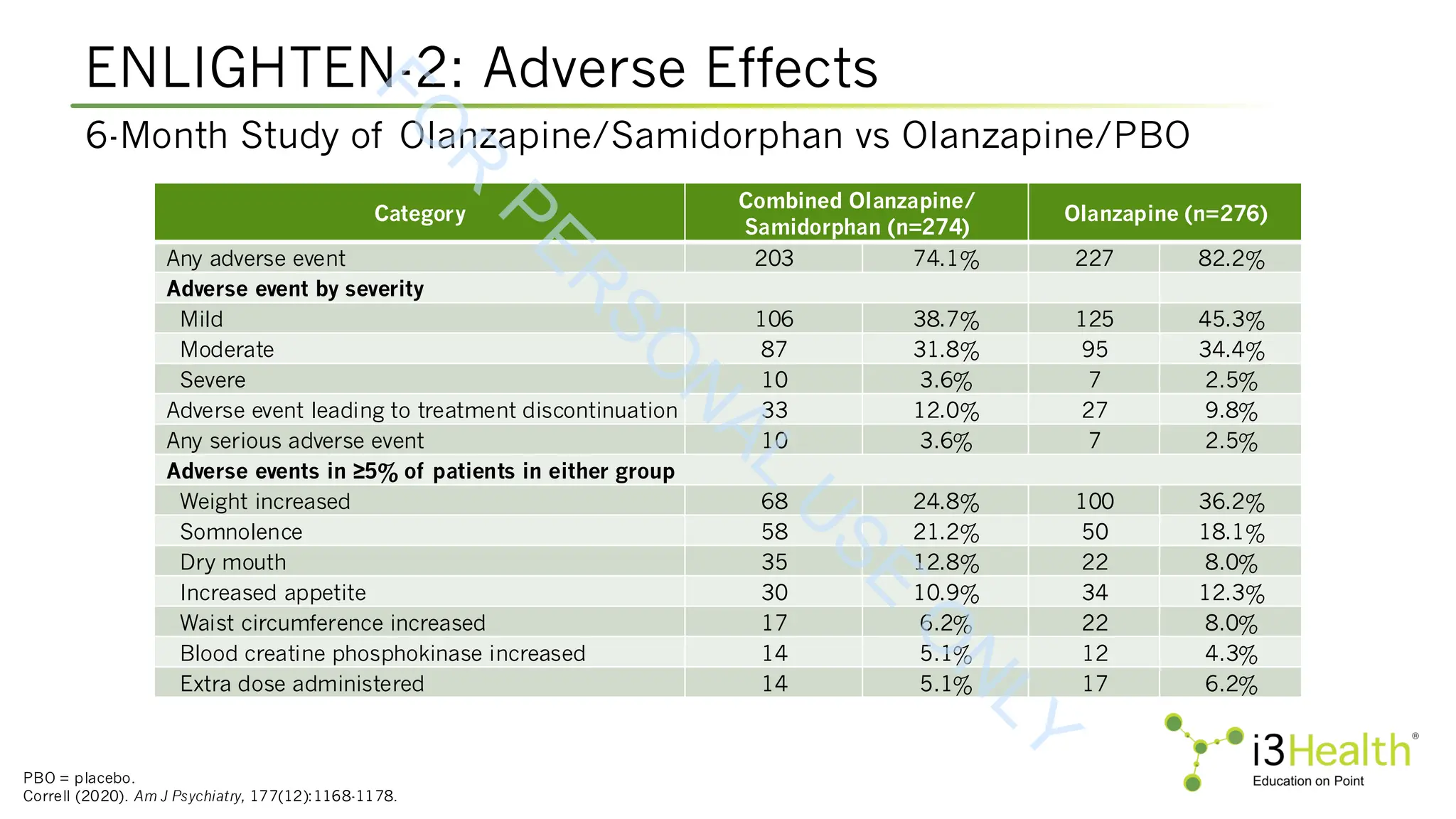
Martin WF, Correll CU, Weiden PJ, et al (2019). Mitigation of olanzapine-induced weight gain with samidorphan, an opioid antagonist: a randomized double-blind phase 2 study in
patients with schizophrenia. Am J Psychiatry, 176(6):457-467. DOI:10.1176/appi.ajp.2018.18030280.
F
O
R
P
E
R
S
O
N
A
L
U
S
E
O
N
L
Y](https://image.slidesharecdn.com/244schizpresentationforslideshare-251006132442-78e94853/75/Addressing-Positive-Negative-and-Cognitive-Symptoms-of-Schizophrenia-New-and-Emerging-Strategies-for-Success-77-2048.jpg)

![References (cont.)
Perlstein I, Gomeni R, Bilgraer R, et al (2024). Population pharmacokinetic modeling following administration of olanzapine for extended-release injectable suspension (TV-44749)
for subcutaneous use to support dose selection for phase 3 clinical trial (SOLARIS). [Oral Presentation]. Presented at the 2024 Schizophrenia International Research
Society 2024 Annual Congress. Abstract 54.
Pillinger T, Howes OD, Correll CU, et al (2023). Antidepressant and antipsychotic side-effects and personalized prescribing: a systematic review and digital tool development.
Lancet Psychiatry, 10(11):860-876. DOI:10.1016/S2215-0366(23)00262-6
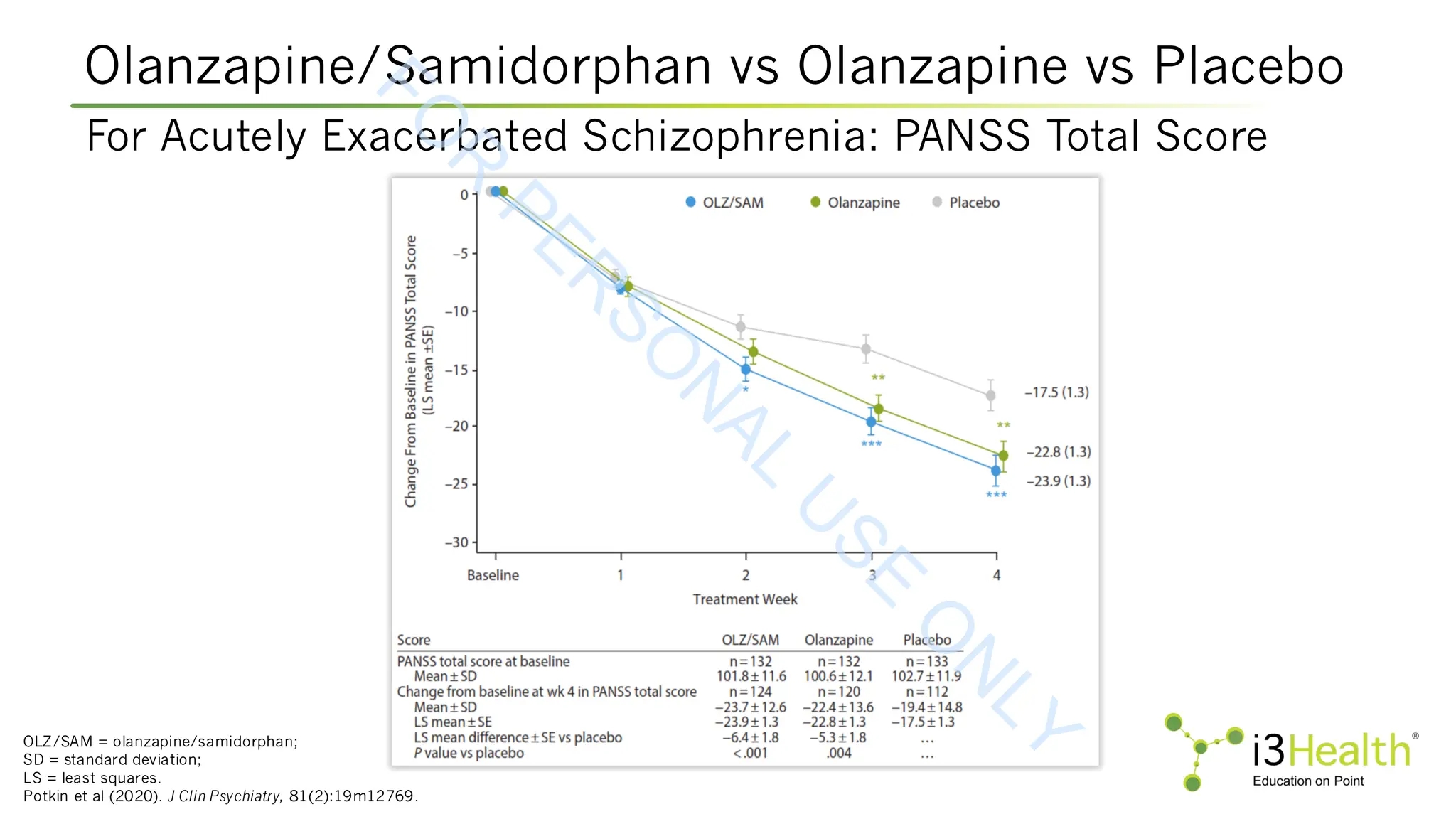
Potkin SG, Kunovac J, Silverman BL (2020). Efficacy and safety of a combination of olanzapine and samidorphan in adult patients with an acute exacerbation of schizophrenia:
outcomes from the randomized, phase 3 ENLIGHTEN-1 study. J Clin Psychiatry, 81(2):19m12769. DOI:10.4088/JCP.19m12769
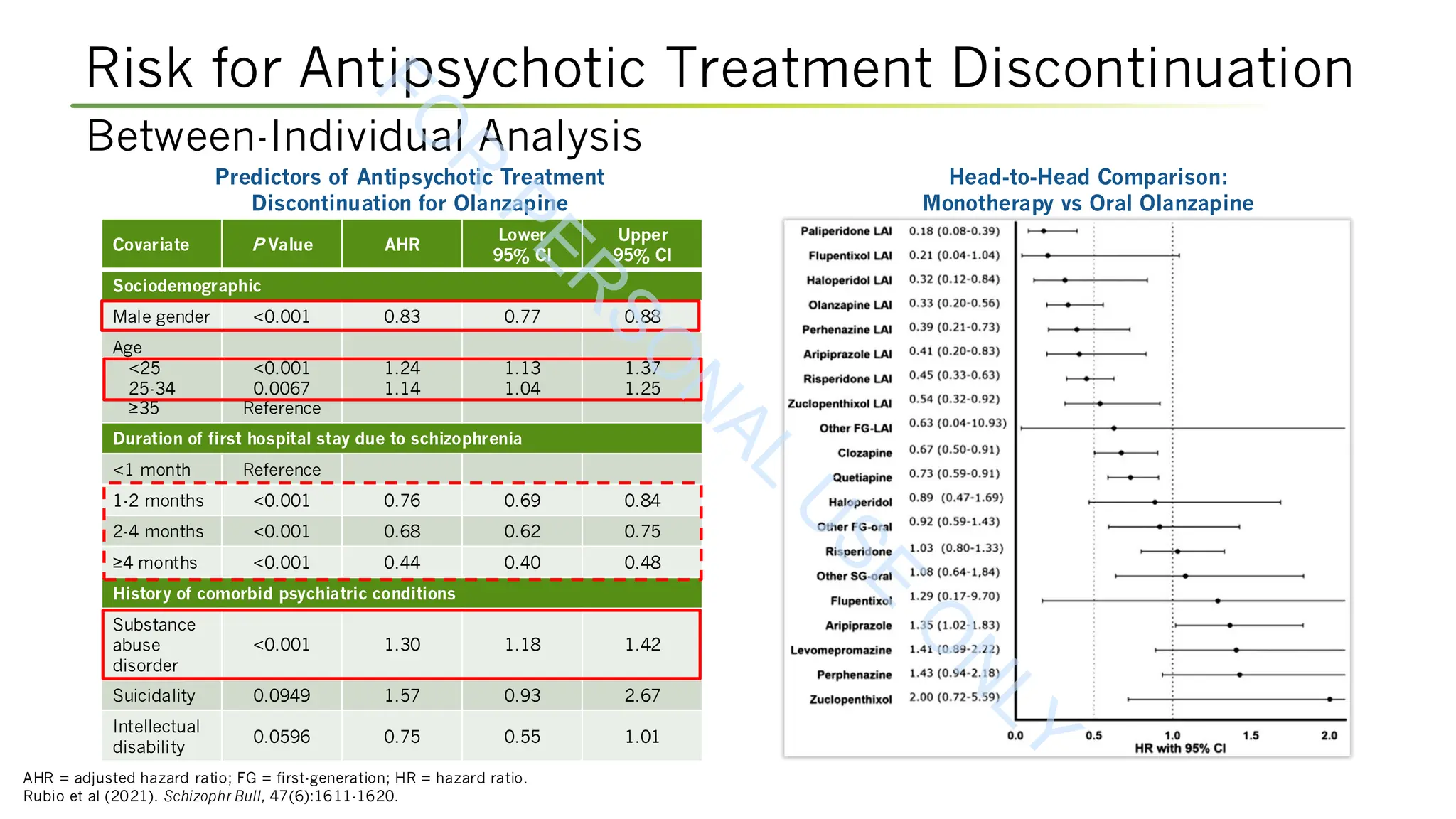
Rubio JM, Taipale H, Tanskanen A, et al (2021). Long-term continuity of antipsychotic treatment for schizophrenia: a nationwide study. Schizophr Bull, 47(6):1611-1620.
DOI:10.1093/schbul/sbab063
Sabbe B (2012). Cognitive and motor disorders in schizophrenia. Proc Belg Roy Acad Med, 1:77-88.
Salzmann-Erikson M & Sjödin M (2018). A narrative meta-synthesis of how people with schizophrenia experience facilitators and barriers in using antipsychotic medication:
implications for healthcare professionals. Int J Nurs Stud, 85:7-18. DOI:10.1016/j.ijnurstu.2018.05.003
Shulman K et al (2024). Monthly olanzapine extended-release injectable suspension (TV-44749) for subcutaneous use leads to early, consistent symptom improvements through
Week 8 in adults with acute exacerbation of schizophrenia: phase 3 SOLARIS trial. [Poster presentation]. Presented at Psych Congress, 2024. Poster 96.
Silverman BL, Martin W, Memisoglu A, et al (2018). A randomized, double-blind, placebo-controlled proof of concept study to evaluate samidorphan in the prevention of
olanzapine-induced weight gain in healthy volunteers. Schizophrenia Res, 195:245-251. DOI:10.1016/j.schres.2017.10.014
Street Jr. RL, Makoul G, Arora NK & Epstein RM (2009). How does communication heal? Pathways linking clinician-patient communication to health outcomes. Patient Educ Couns,
74(3):295-301. DOI:10.1016/j.pec.2008.11.015
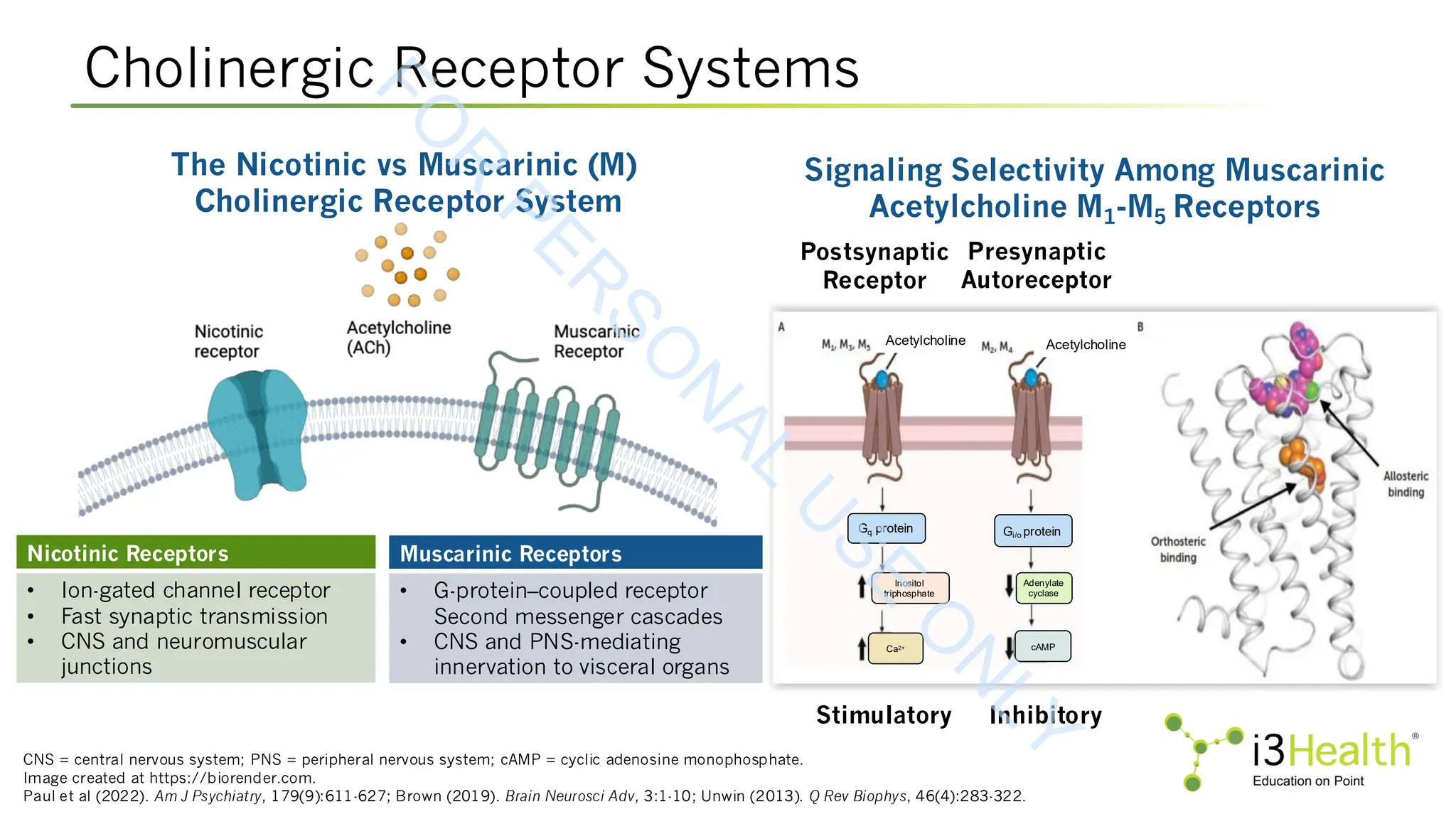
Unwin N (2013). Nicotinic acetylcholine receptor and the structural basis of neuromuscular transmission: insights from Torpedo postsynaptic membranes. Q Rev Biophys,
46(4):283-322. DOI:10.1017/S0033583513000061
Ventriglio A, Gentile A, Bonfitto I, et al (2016). Suicide in the early stage of schizophrenia. Front Psychiatry, 7:116. DOI:10.3389/fpsyt.2016.00116
Weiden PJ (2007). Understanding and addressing adherence issues in schizophrenia: from theory to practice. J Clin Psychiatry, 68(suppl_14):14-19
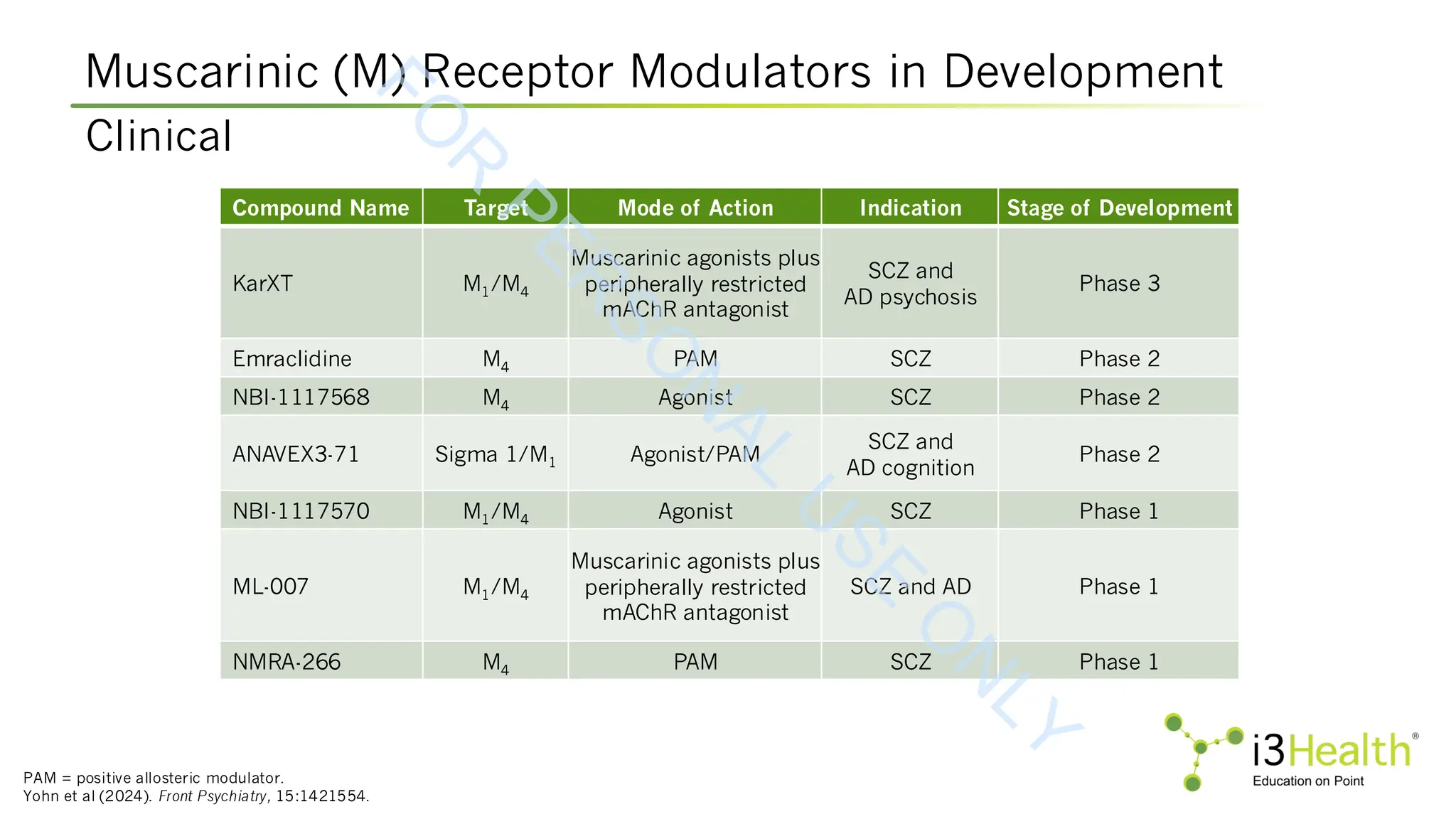
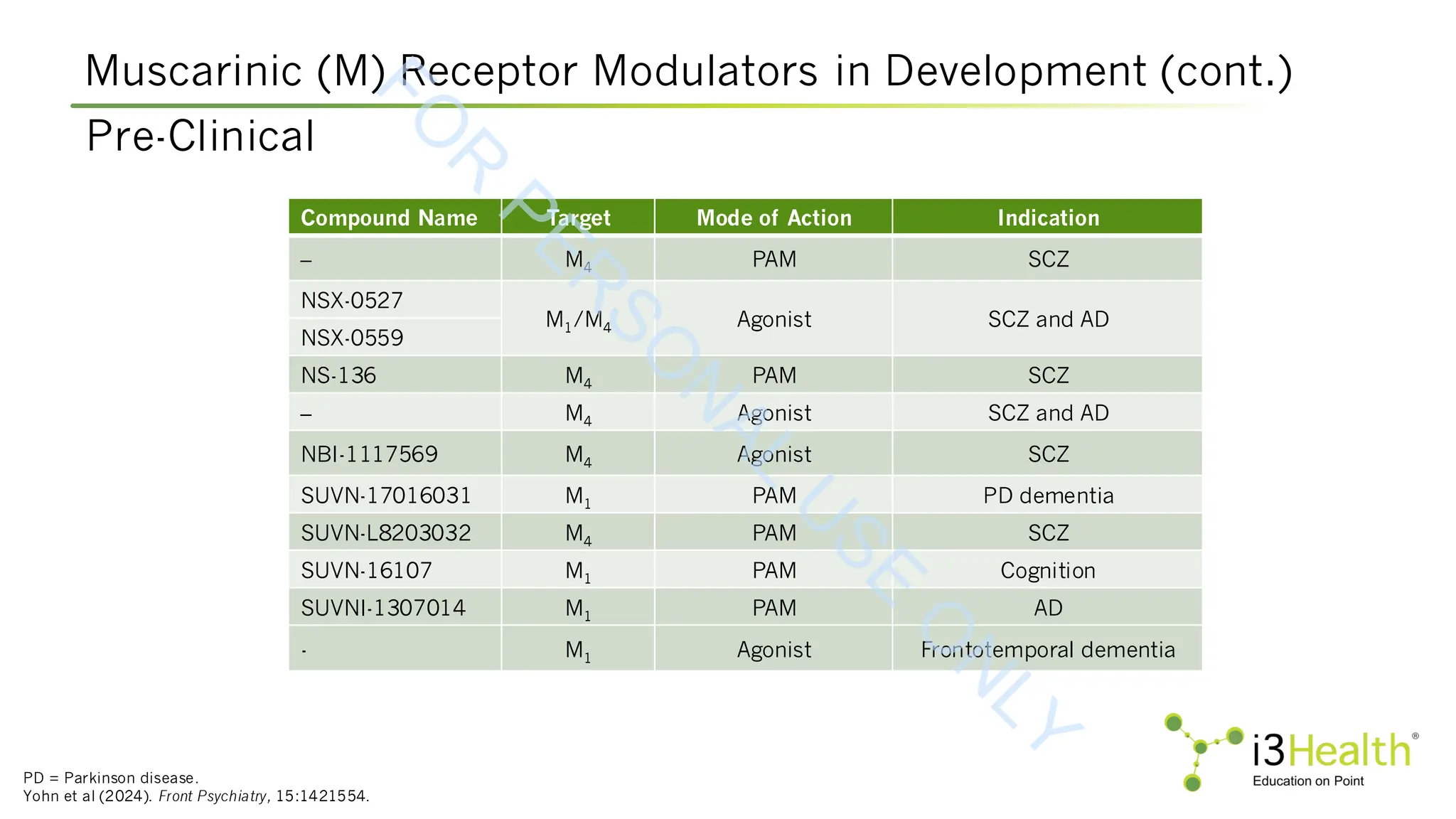
Yohn SE, Harvey PD, Brannan SK, Horan WP (2024). The potential of muscarinic M1 and M4 receptor activators for the treatment of cognitive impairment associated with
schizophrenia. Front Psychiatry, 15:1421554. DOI:10.3389/fpsyt.2024.1421554
F
O
R
P
E
R
S
O
N
A
L
U
S
E
O
N
L
Y](https://image.slidesharecdn.com/244schizpresentationforslideshare-251006132442-78e94853/75/Addressing-Positive-Negative-and-Cognitive-Symptoms-of-Schizophrenia-New-and-Emerging-Strategies-for-Success-79-2048.jpg)
