This document contains instructions and information for a lesson on acids and bases:
1. It includes directions for students to complete various tasks like turning in work, taking a quiz, and accessing online resources.
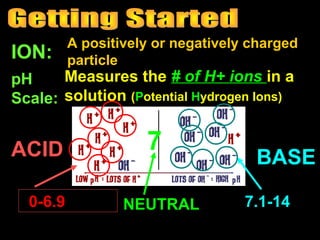
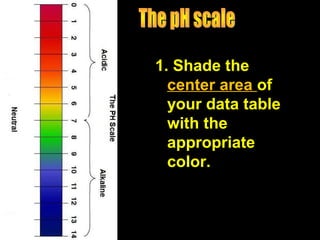
2. Key concepts about acids and bases are defined, like what an ion and the pH scale are. Acids are described as having more hydrogen ions and being associated with certain colors.
3. Students are provided questions to answer in a group about acids and bases, which will then be checked with an answer key.