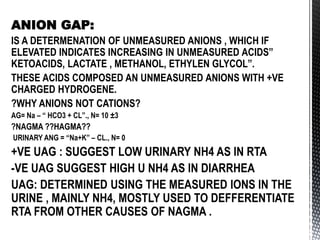
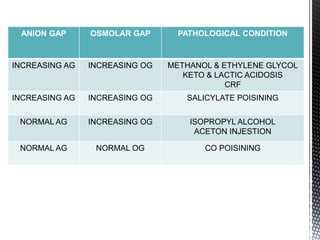
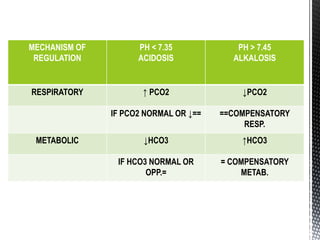
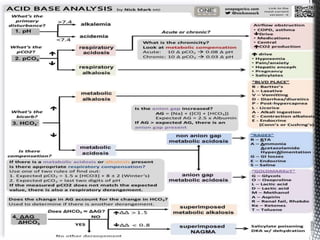
This document summarizes acid-base physiology and interpretation of arterial blood gases. It defines the Henderson-Hasselbalch equation and describes how the respiratory and renal systems regulate pH. Primary respiratory and metabolic acid-base disorders are explained. The anion gap, urinary anion gap, and osmolar gap are defined and their clinical significance discussed. Various acid-base imbalances including respiratory acidosis/alkalosis and metabolic acidosis/alkalosis are outlined along with their causes. Steps for interpreting arterial blood gases and examples of analysis are provided.