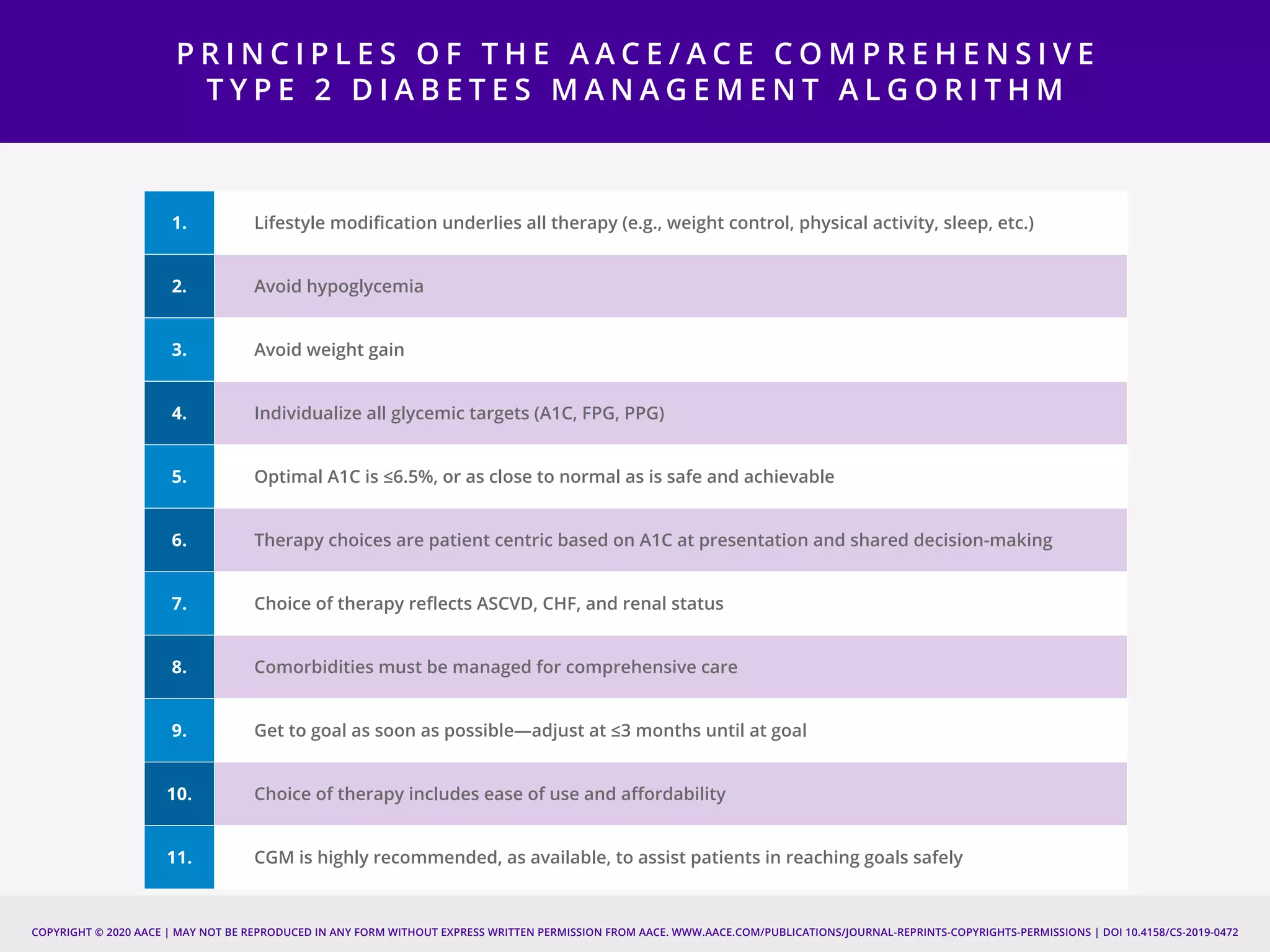
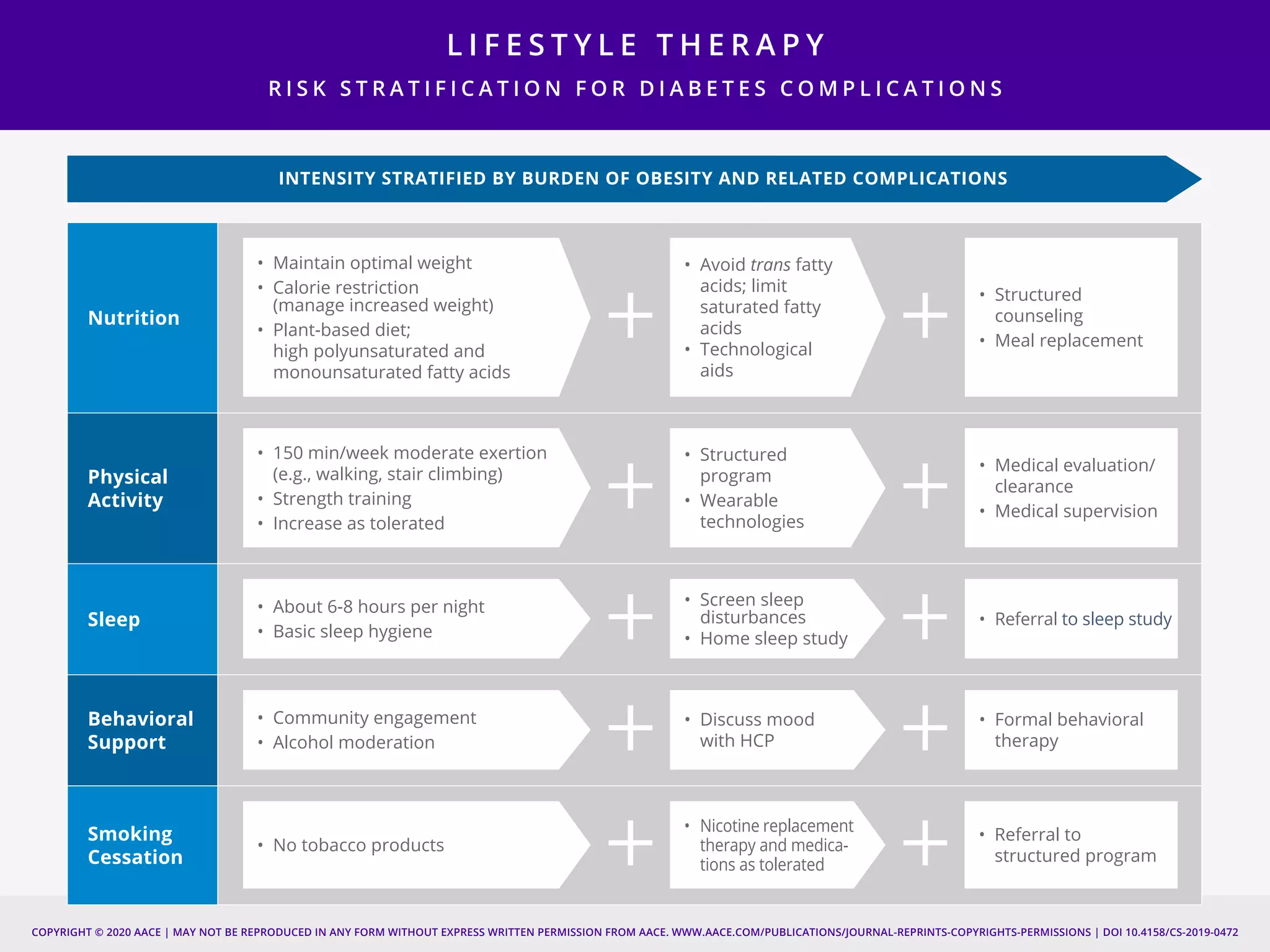
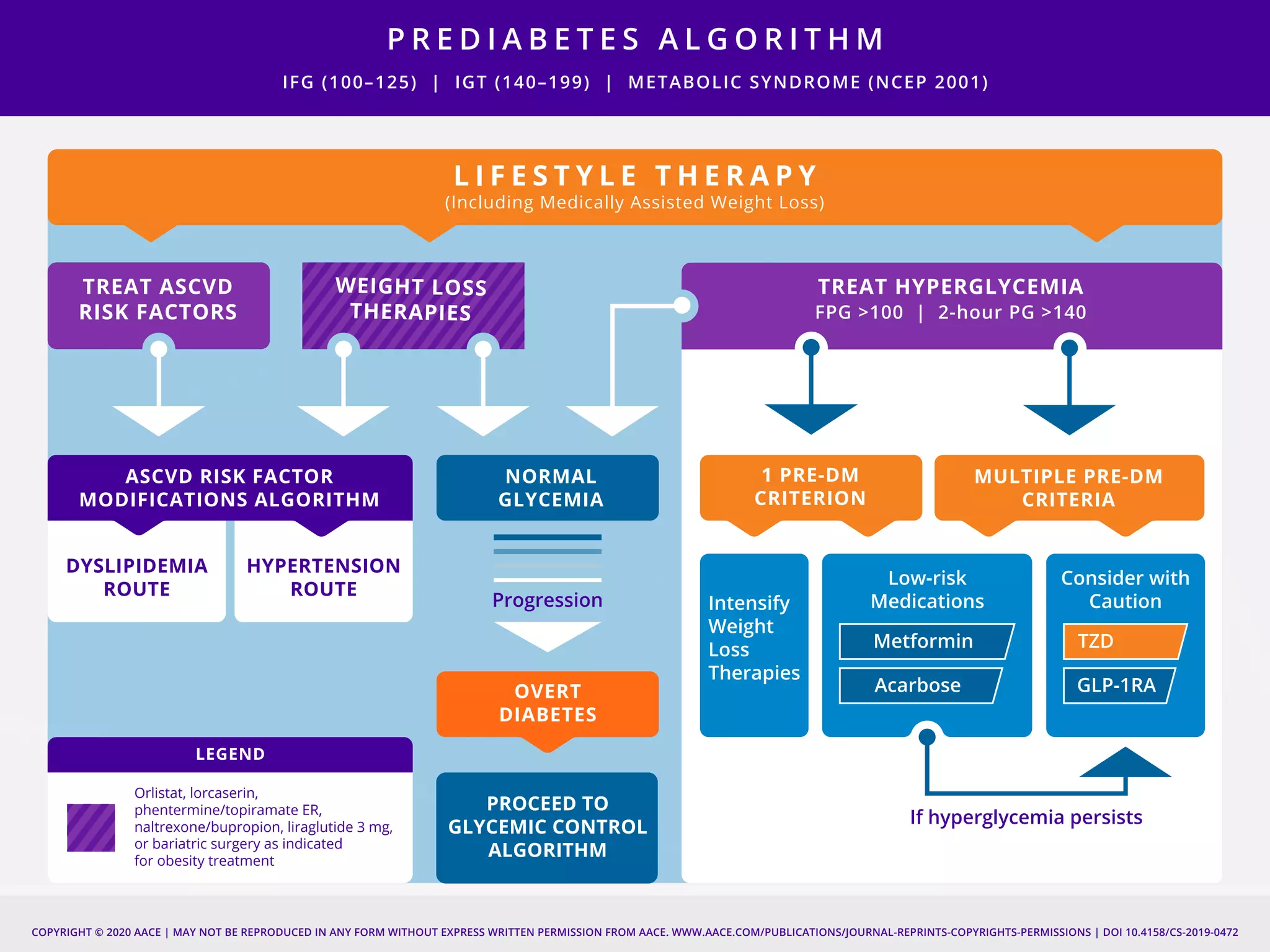
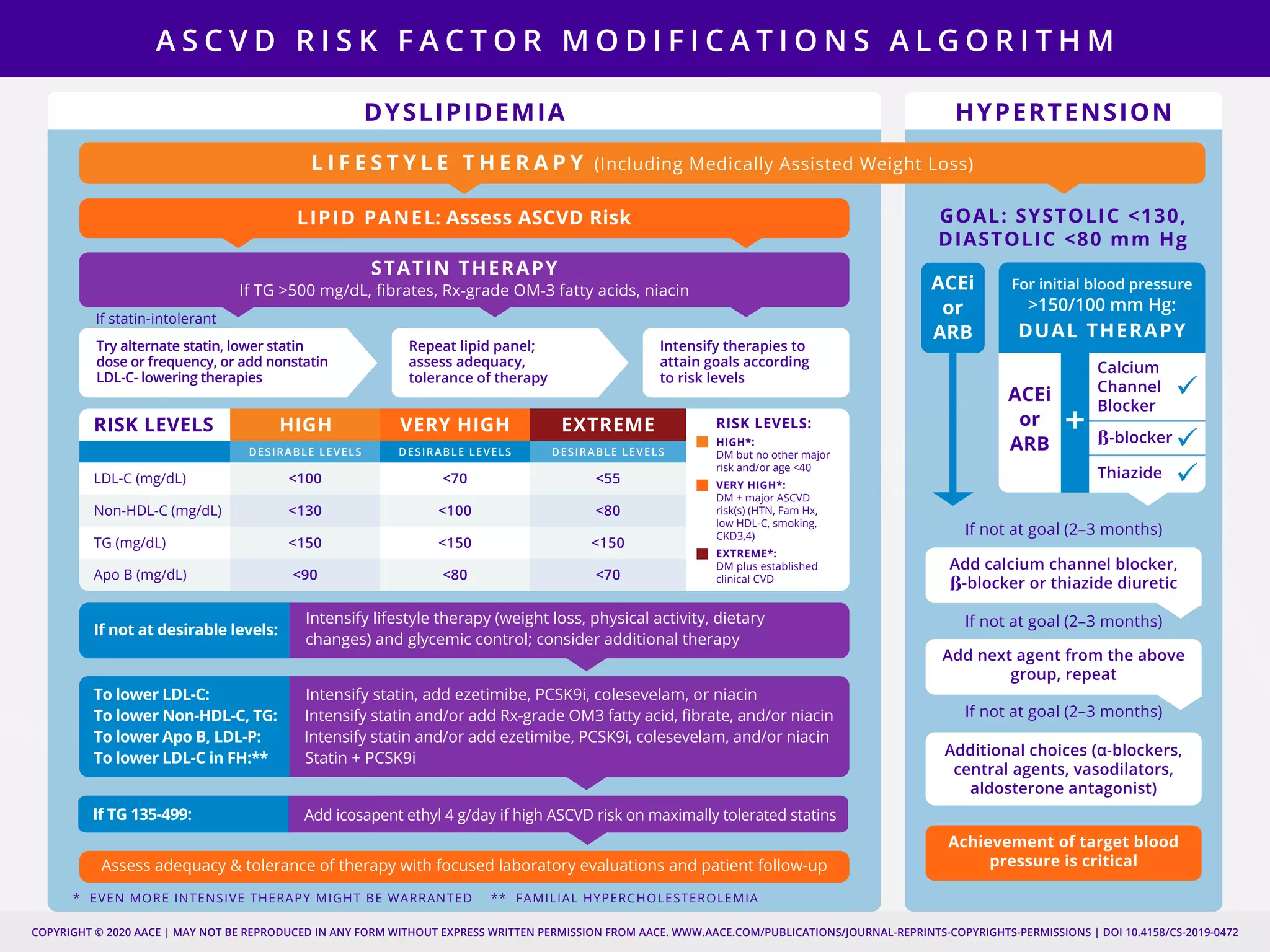
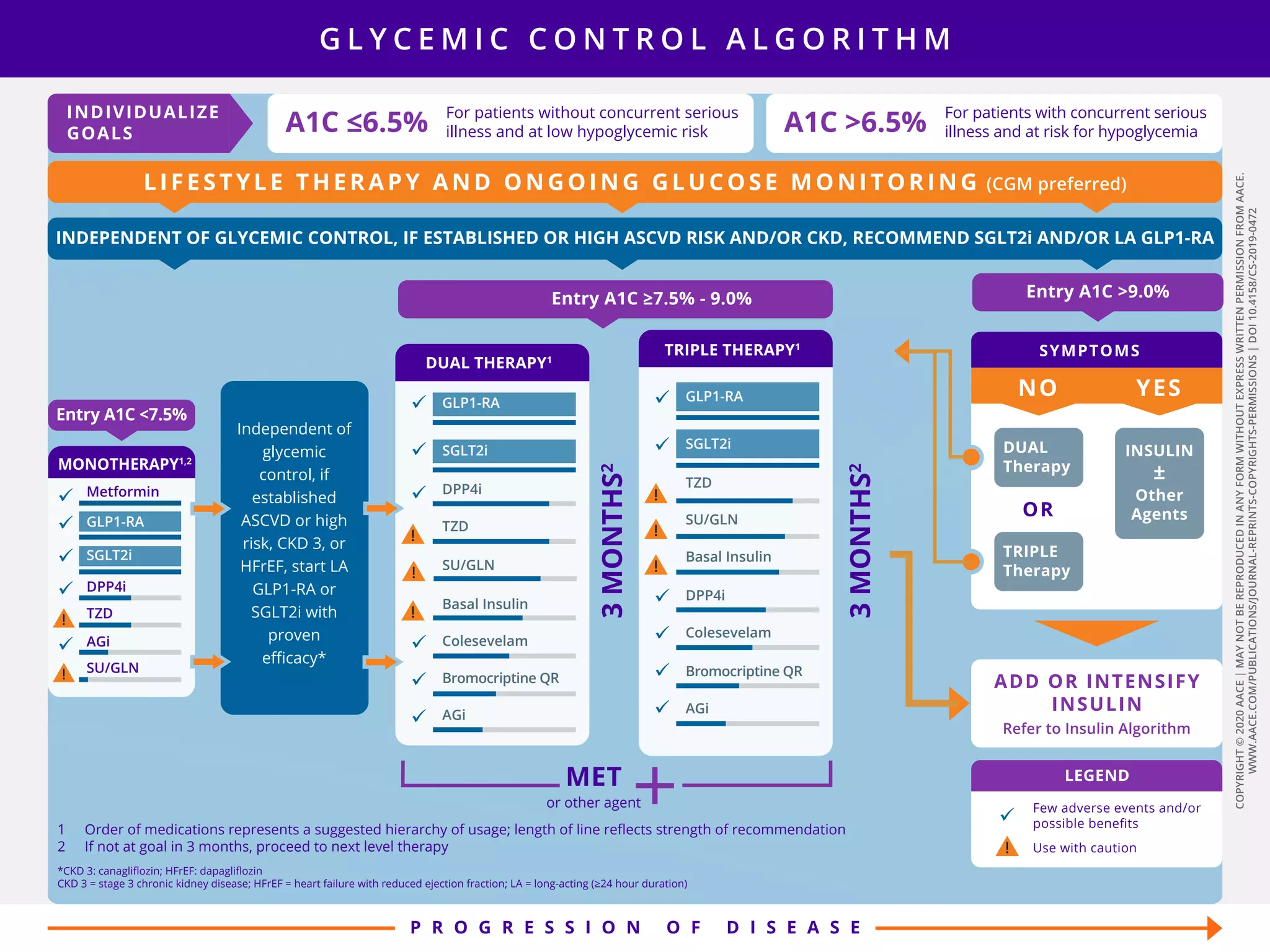
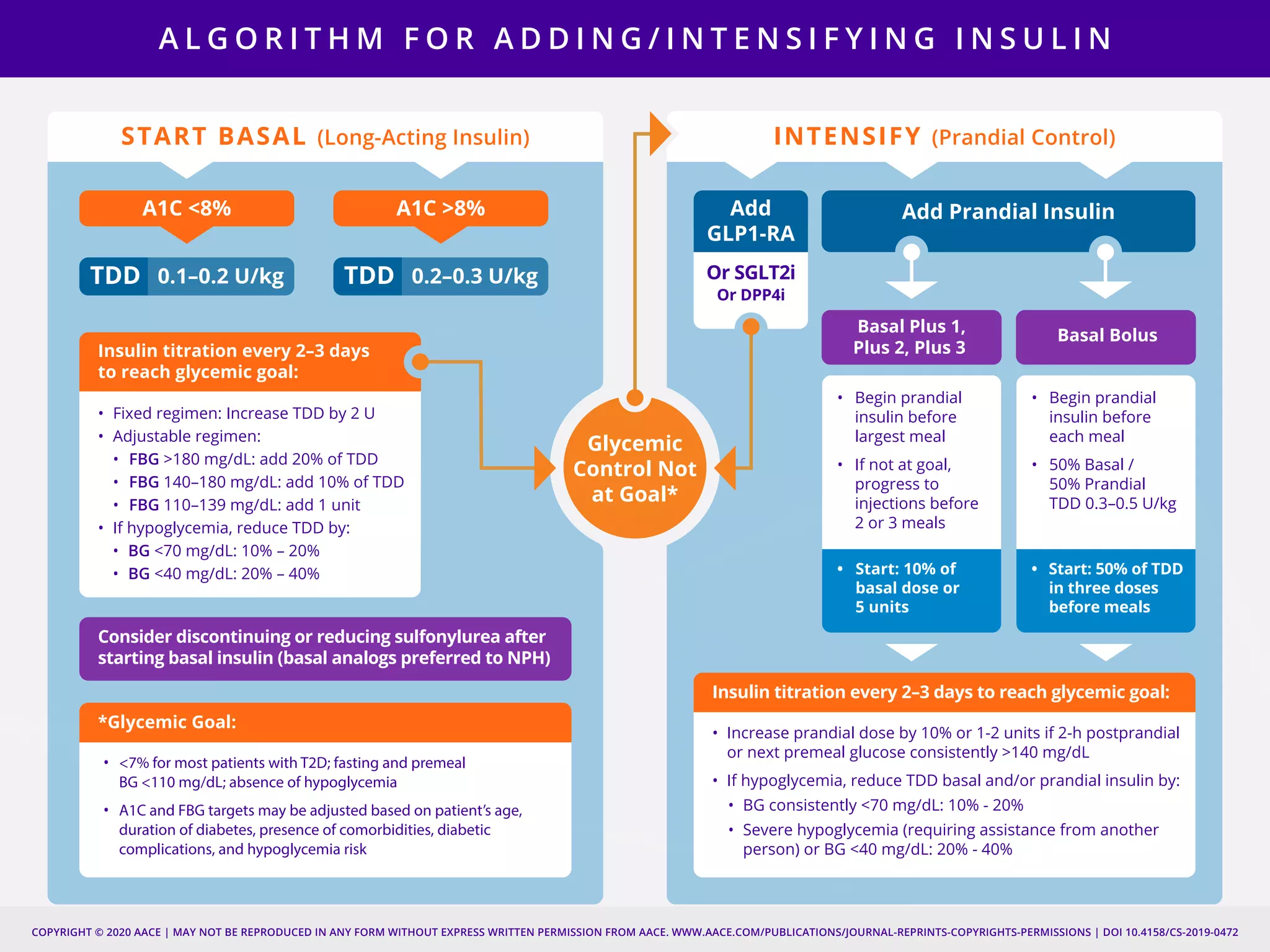
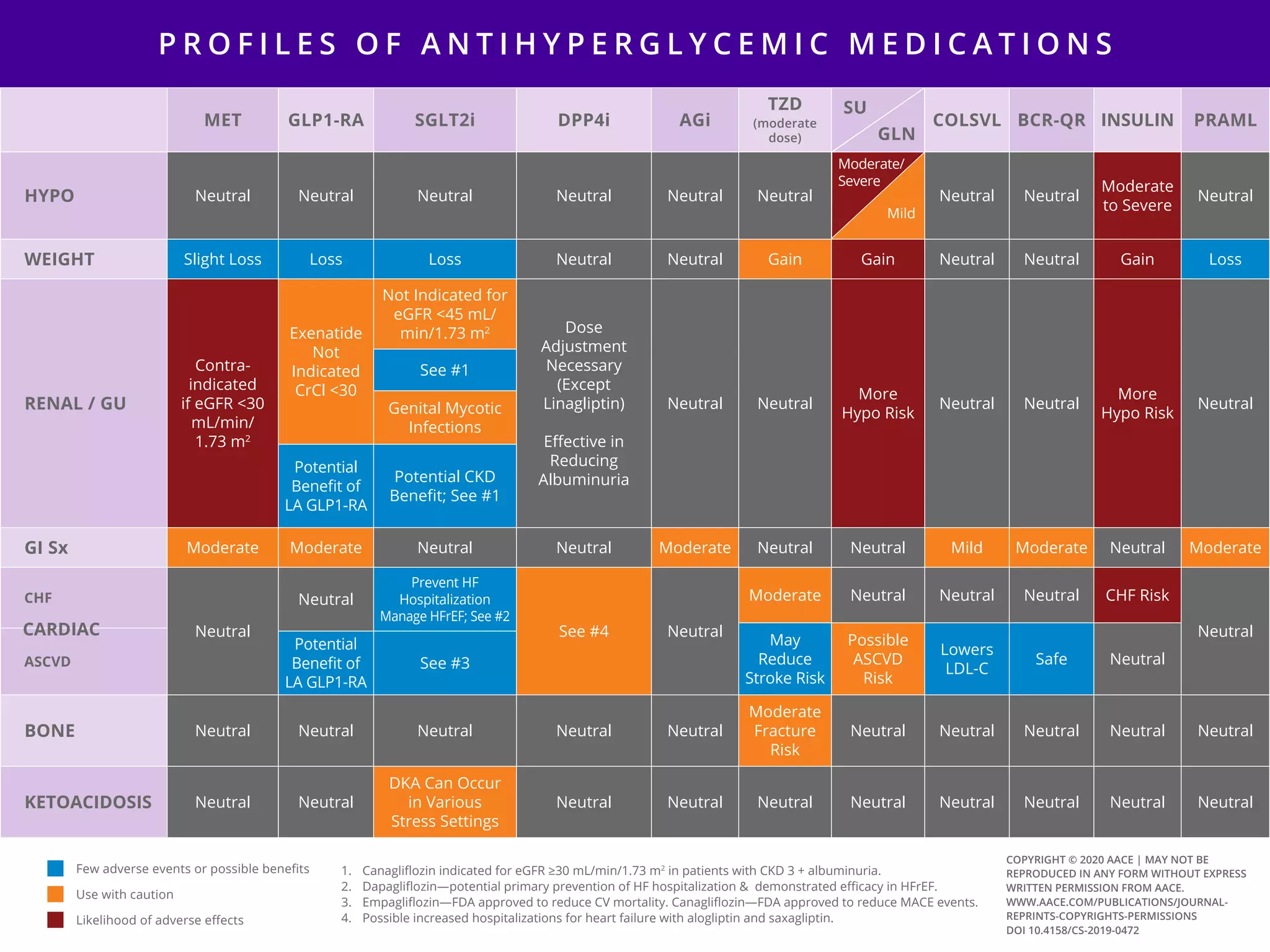
This document provides guidelines for the comprehensive management of type 2 diabetes. It includes sections on lifestyle therapy, risk stratification for diabetes complications, prediabetes treatment, cardiovascular risk factor modification, glycemic control, and adding or intensifying insulin therapy. The guidelines emphasize individualizing treatment based on each patient's needs and circumstances. Lifestyle modification is emphasized as the foundation for all treatment. Medications, including insulin, are recommended to control blood sugar and cardiovascular risk factors based on each patient's clinical profile and treatment goals.