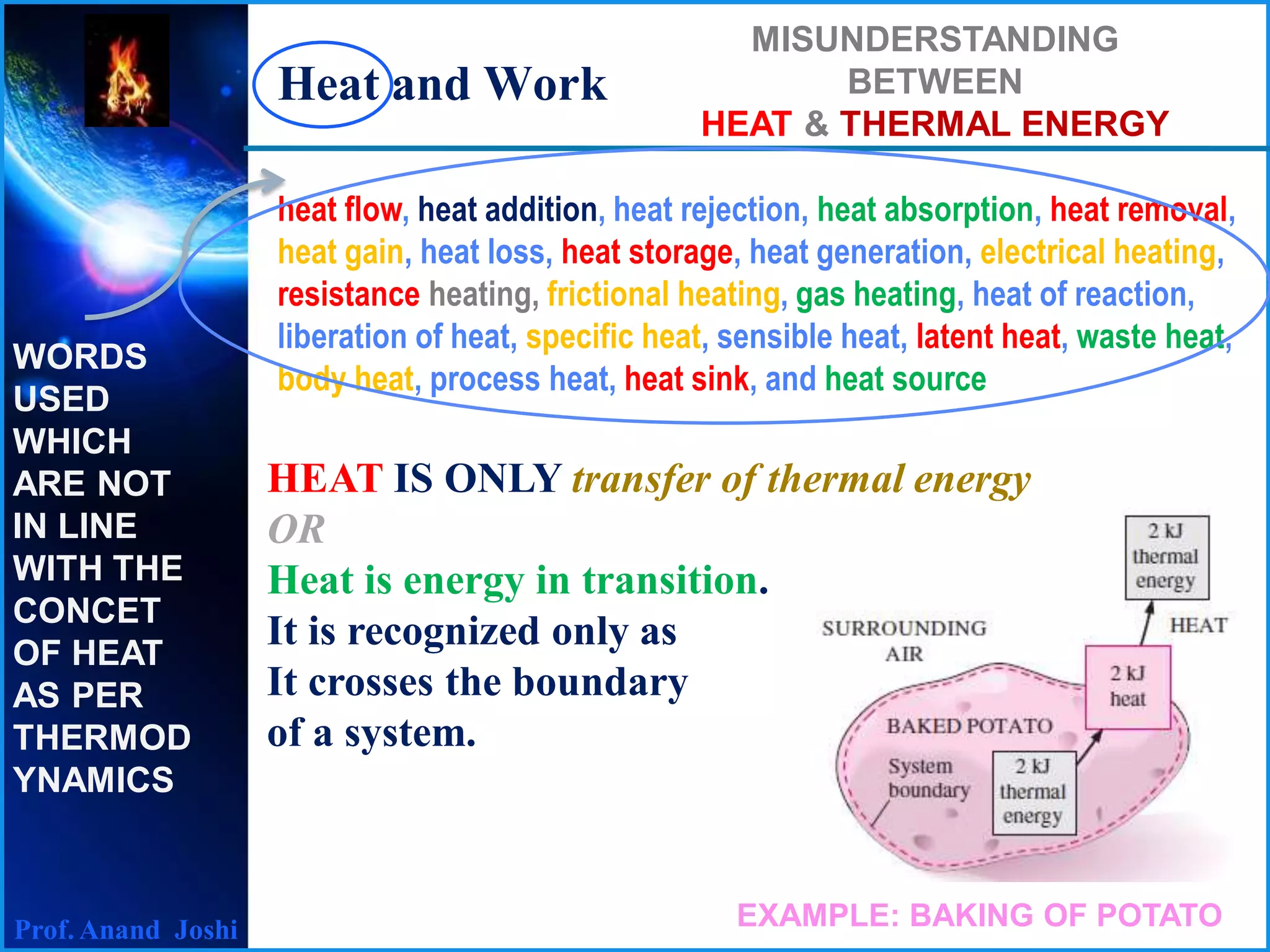
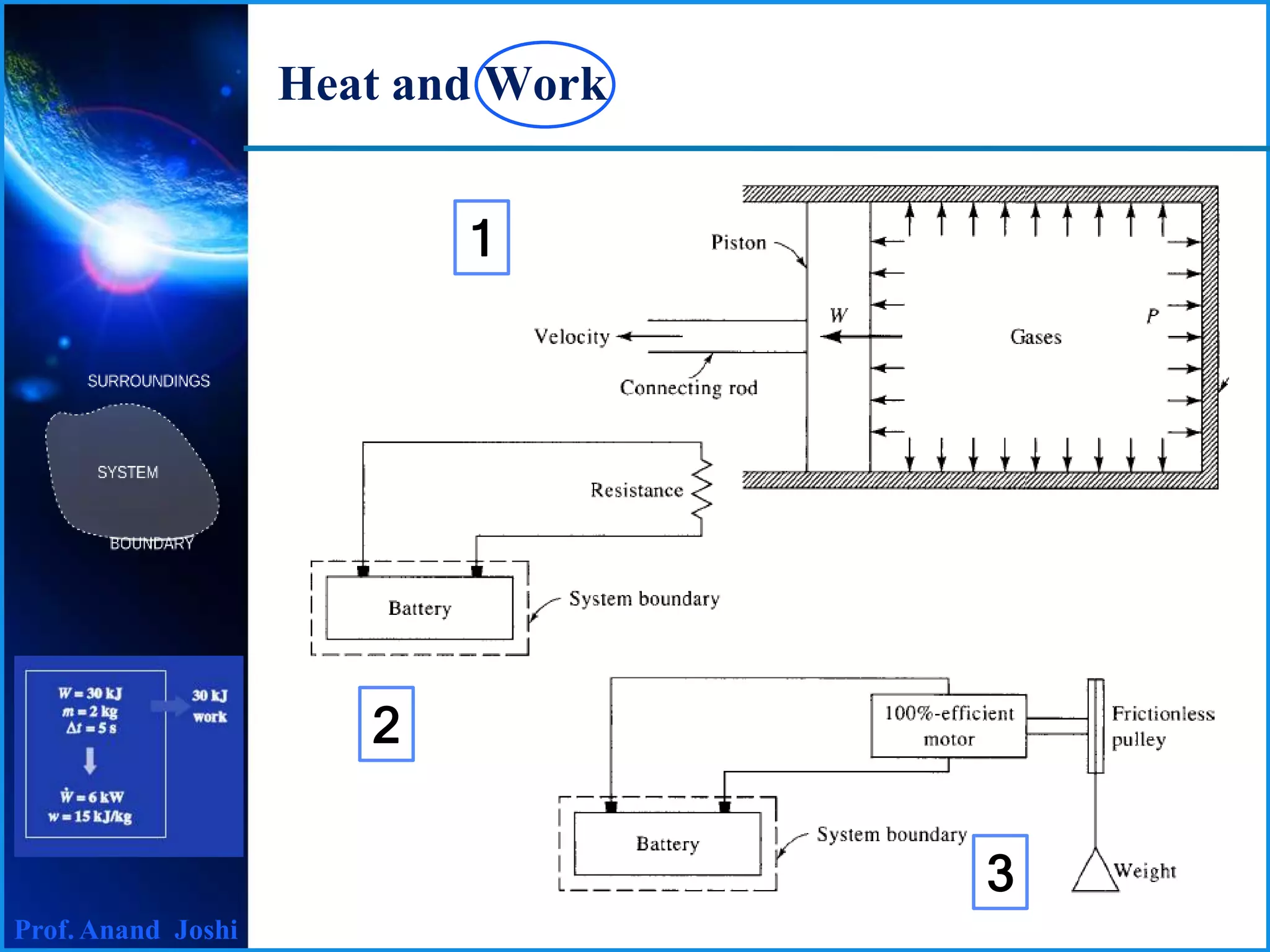
1. Heat and work are both forms of energy transfer between systems or a system and its surroundings.
2. Heat is recognized as energy transfer due solely to a temperature difference, while work is the energy transfer associated with a force acting through a distance.
3. Both heat and work are path functions that depend on the process followed between initial and final states, rather than just the initial and final thermodynamic states alone.