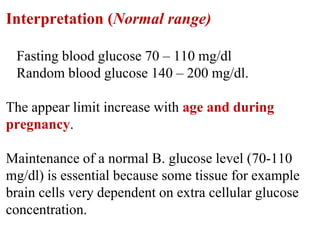
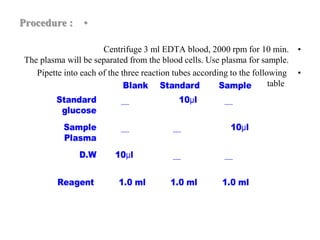
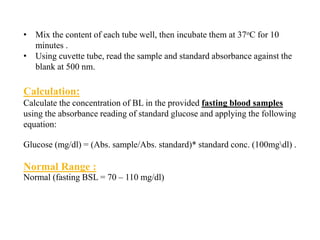
This document provides instructions for determining serum glucose levels using a glucose oxidase method. It begins with background information on glucose and its role in the body. It then describes the two main methods for collecting blood samples and discusses normal and abnormal glucose ranges. The principle of the glucose oxidase method is explained, where glucose is oxidized to produce a colored complex that can be measured. Finally, it provides the procedure for the test, including sample preparation and calculations to determine glucose concentration in mg/dL.