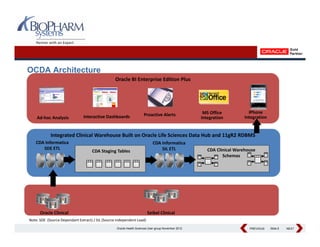
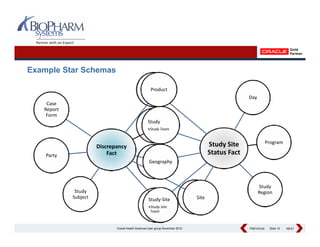
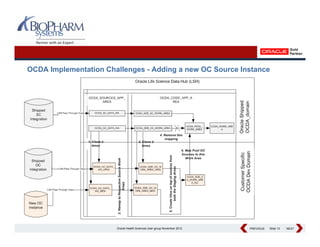
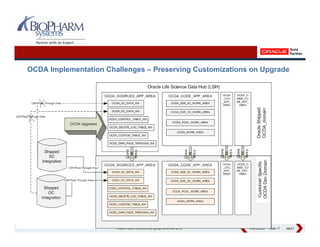
This presentation discusses the practical considerations of implementing and customizing Oracle Clinical Development Analytics (OCDA). It provides an overview of OCDA and its architecture, shows some example star schemas, and discusses challenges such as handling multiple Oracle Clinical instances, integrating additional data sources, and preserving customizations across releases.