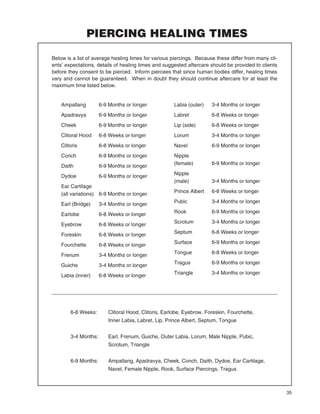
The Association of Professional Piercers has updated their aftercare brochures and procedures manual.
The new brochures provide clearer cleaning instructions, emphasize gentle aftercare, include more information on jewelry issues, and cover facial piercings. The manual is intended as a health and safety reference for piercers, health officials, and the public. It outlines infection control protocols, equipment sterilization, skin preparation, aftercare, and ethics.


![THE ASSOCIATION OF PROFESSIONAL PIERCERS MANUAL
U.S. EDITION CONTENTS
WHAT IS THE APP? ............................................................................................................................. 1
INTRO TO MICROBIOLOGY FOR THE PIERCER............................................................................... 5
INFECTION CONTROL......................................................................................................................... 9
STERILE CHART ................................................................................................................................ 14
CLEANING, DISINFECTION AND STERILIZATION .......................................................................... 15
EQUIPMENT ....................................................................................................................................... 19
ENVIRONMENT.................................................................................................................................. 25
SKIN PREPARATION ......................................................................................................................... 29
AFTERCARE........................................................................................................................................31
PIERCING HEALING TIMES .............................................................................................................. 35
JEWELRY............................................................................................................................................ 37
ETHICS AND LEGALITIES ................................................................................................................. 43
COMPLIANCE AND TRAINING ..........................................................................................................49
EMERGENCIES.................................................................................................................................. 53
AFTERWARD...................................................................................................................................... 58
GLOSSARY......................................................................................................................................... 59
UNDERSTANDING MSDS.................................................................................................................. 63
APPENDIX A - FULL TEXT OF BLOODBORNE PATHOGENS STANDARD 1910.1030.................. 65
APPENDIX B - HEPATITIS B VACCINE DECLINATION (MANDATORY) ......................................... 77
APPENDIX C - APPLICATIONS FOR MEMBERSHIP........................................................................ 79
This manual was first published in 1998 and was revised in 2002 and 2005.
Previous edition credits are extended to:
Gahdi Elias, Allen Falkner, Tracy Faraka, Kent Fazekas, Michaela Gray, Drew Lewis, Cheyenne Morrisson, David Vidra,
Dr. Jack Ward [Original Edition]; and to: Elayne Angel, Scott Brewer, Steve Joyner, Lisa Lystad, M.D., Patrick McCarthy,
Sky Renfro, Bethra Szumski, April Williams-Warner, Dr. Jack Ward [2002 Edition].
Contributors to the 2005 edition include: Elayne Angel, Alicia Cardenas, Luis Garcia, Phish Goldblatt, Schane Gross, April
Johnson, Jason King, Paul King, Megg Mass, Christina Shull, Crystal Sims, Bethra Szumski, James Weber, and the intrepid
Caitlin McDiarmid.
Some cover photos courtesy of Evolution Body Piercing, Inc.
Cover design by Paul Romano, www.workhardened.com
Copyright © 1998, 2002, 2005. All rights reserved. The goal of the Association of Professional Piercers is to circulate vital
health, safety and educational information to the piercing industry, health care workers and the general public. This manual
is copyrighted under Federal Law. Any reproduction of its contents is prohibited without prior written permission. For specific
reprint permissions, please contact us directly.](https://image.slidesharecdn.com/2005manual-130807100017-phpapp02/85/2005-manual-3-320.jpg)













![13
but may well be worth it depending upon a piercer’s
preferences and circumstances. Contact individual
suppliers for samples.
*Adenna, Inc. ©1997-2004.
LATEX SENSITIVITIES
Since the implementation and recommendation of Uni-
versal Precautions by the Centers for Disease Control
and Prevention (CDC) and Occupational Safety and
Health Administration (OSHA) , latex sensitivities have
been on the rise. Estimates suggest 2 - 6% of the gen-
eral population is latex sensitive. Among healthcare
workers this percentage rises to 8 - 12%. Due to the
extensive exposure many people in the healthcare,
emergency service, body art and other industries have
to latex gloves and protective gear, allergic reactions
among these groups are increasingly common and if
unchecked can interfere with continued employment.
Reactions can range from minor rash to life-threaten-
ing respiratory distress. Because latex allergies are
actually sensitization reactions from overexposure to
latex, and because latex is so pervasive in our home
and work environments, prevention through limiting
exposure is crucial. Those who come into frequent
contact with latex through the skin or inhalation be-
come sensitized, and may go on to develop full allergic
reactions. These reactions often occur gradually, but
can also come on quite suddenly. Those who use
gloves at work, have had multiple surgeries, or who
have multiple allergic sensitivities or spina bifida are
especially at risk.
In the piercing studio, many items besides gloves
contain latex. Adhesive tape, rubber bands, some
autoclave wrap, CPR masks, and even the handles
of some tools and covering on ballpoint pens may
contain traces of latex. All of these can trigger reac-
tions. Piercers should understand the types of latex
reaction and how to deal with latex-sensitive clients
and coworkers.
The three types of latex hypersensitivity reactions that
occur are classified in order of severity as Irritant Contact
Dermatitis, Type IV Reaction and Type I Reaction.
Irritant Contact Dermatitis
This type of reaction is actually not an allergy, but rather
a surface irritation caused by excessive handwashing,
harsh soaps, chemicals, hand sanitizers and/or glove
powder. It can appear as overly dry, cracked, sore or
flaky skin and rash. To avoid or relieve contact derma-
titis, switch to milder products, avoid powdered gloves,
and use a soothing hand sealant after washing. Avoid
hand sanitizer or products that increase irritation and be
aware that cracked skin is open to latex, chemical and
pathogenic penetration. Although contact dermatitis
is not serious in itself, it can lead to further problems
if not cared for.
Type IV Reaction: Chemical Protein
Hypersensitivity
Type IV is a delayed reaction to an allergen that usually
appears 48 to 96 hours after contact. Most people with
Type IV hypersensitivity experience some form of der-
matitis, such as rash, scales, inflammation, or eczema.
Reactions may also include conjunctivitis, runny nose
or asthma-like symptoms from airborne detritus. It is
generally believed that this type of response is due to
the chemicals used in manufacturing the rubber and
not due to the latex itself.
Type I Reaction: Latex Protein Sensitivity
This is an immediate and systemic response to latex
proteins. People with this type of reaction may have
intense burning and irritation at the contact site, hives
within 5 to 60 minutes, itchy eyes, swelling of lips and
tongue, abdominal pain, asthma, nausea, and in severe
cases death from anaphylactic shock.
What To Do
For their own health and that of their clients, it is im-
perative that professional piercers take these health
concerns seriously. If you suspect you have a latex
sensitivity, consult a doctor or allergist. Because se-
vere reactions can develop suddenly and continued
exposure increases this risk, cease all contact with
latex products. This will often mean making the entire
studio latex-free. The use of low-chemical, low-protein,
low-endotoxin powder free synthetic gloves (such as
nitrile) is recommended in suspected cases of all three
types of hypersensitivity. Question all clients regarding
latex sensitivity prior to piercing and use only non-latex
materials on clients with a history of sensitivity. Hand
sealants, non-latex gloves and avoidance of chemical
triggers may ease dermatitis and Type IV Reactions.
Medications and allergy therapies are available to mini-
mize some symptoms of Type I Reactions, but there is
no clinically recognized treatment or cure.
For more information
NIOSH has issued an Alert, Preventing Allergic Reac-
tions to Natural Rubber Latex in the Workplace (DHHS
[NIOSH] Publication No. 97-135). Free copies are
available from the NIOSH Publications Office: 1-800-
35-NIOSH (1-800-356-4674).](https://image.slidesharecdn.com/2005manual-130807100017-phpapp02/85/2005-manual-17-320.jpg)