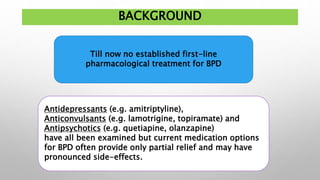
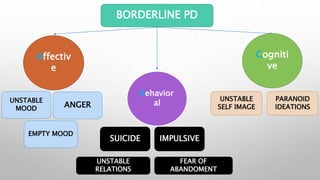
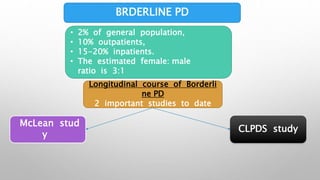
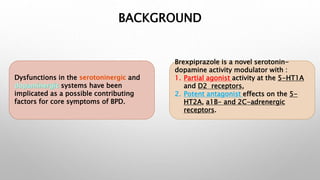
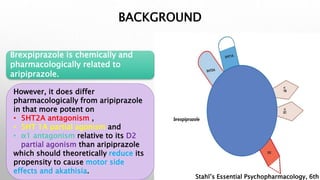
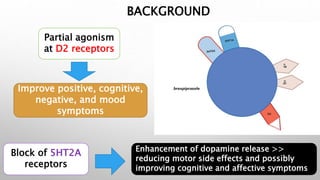
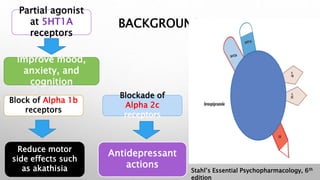
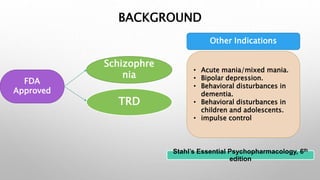
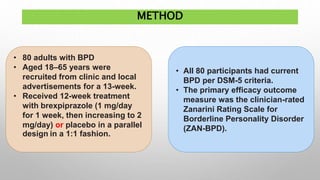
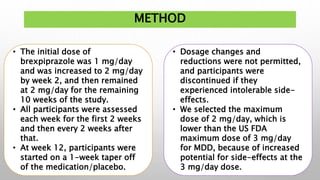
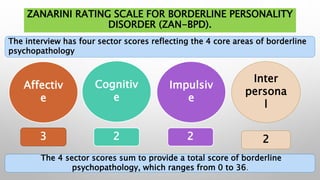
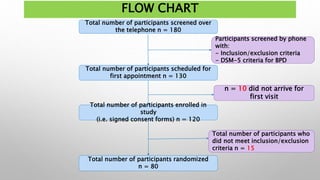
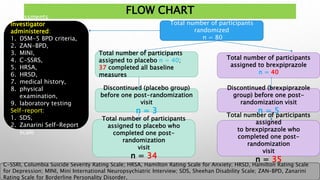
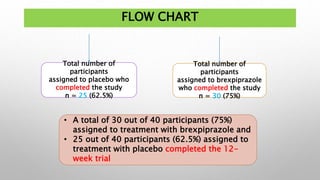
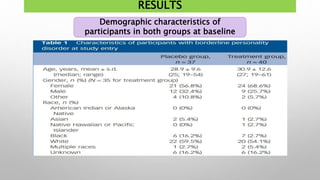
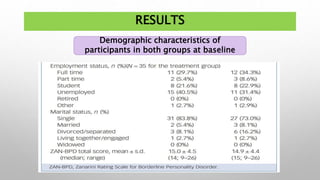
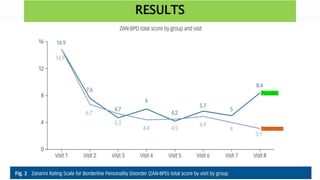
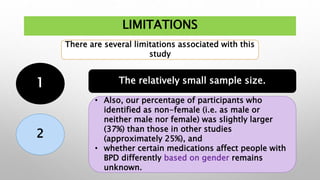
This study examined the efficacy and safety of brexpiprazole compared to placebo for the treatment of borderline personality disorder (BPD) in adults. Eighty adults with BPD were randomized to receive either brexpiprazole or placebo for 12 weeks. The primary outcome was change in scores on the Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD). Results showed that brexpiprazole was associated with greater reductions in ZAN-BPD scores compared to placebo. Brexpiprazole was generally well tolerated, with 75% of those receiving brexpiprazole completing the study compared to 62.5% of those receiving placebo. This suggests that brexpip