Biochemical and clinical aspects of collagen
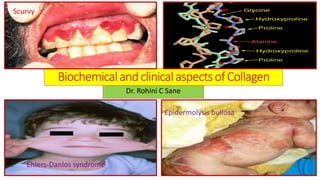
- 1. BiochemicalandclinicalaspectsofCollagen Dr. Rohini C Sane Epidermolysis bullosa Scurvy Ehlers-Danlos syndrome
- 2. Biochemical aspects of Collagen ❖Biochemical aspects of Collagen: 1. Most abundant fibrous protein(major macromolecules) in human body :70 kg body weight→ 12-14 kg of total protein→5kg of Collagen (1/3 of total protein) 2. Main Component of : connective tissue, skin(70%) ,bone (90%) tendon(85%), cartilage ,teeth and liver(4%) 3. Synthesized by fibroblast in connective tissue and osteoblasts in bone 4. Made up of small fibrils → tropocollagen( fundamental units ) containing 3 polypeptide chains each of them in left-handed helix with 3 amino acid per turn. 5. rich in Glycine and rare amino acids like hydroxyproline, hydroxylysine 6. Cysteine and Tryptophan absent 7. have a triple helical secondary structure and rich in helix destabilizing amino acids (Glycine ,Proline and Hydroxyproline). These amino acids prevent the formation of the usual - helical and - pleated structure. Instead it forms a triple helical secondary structure.
- 3. Triple stranded helix Structure of Collagen • Collagenhas3 polypeptidechainswoundarounditself. Eachpolypeptidechainsubunitis calledalpha-chains. EachofAlpha-chain is twistedintoleft-handedhelix ofthreeresidues perturncomparedwith 3.6forright handedalpha-helix.Threeoftheseleft– handedhelices arethenwoundtoright-handedsuperhelixtoformastiffrodlike molecule(Triplehelical secondarystructure). • Itisrich in helixdestabilizingaminoacids(Glycine ,ProlineandHydroxyproline).Theseamino acids preventtheformationoftheusual- helicaland-pleatedstructure.Instead,it formsa triplehelicalsecondarystructure. • Every3rdresidueis Glycineandtheonlyaminoacidthatcanfitintothetriplestrandedhelix. • QuarterstaggeredtriplestrandedhelixofCollagenis stabilizedbythestericrepulsionof ringshydroxyproline andhydrogenbondsbetweenthem. • Triplehelicalsecondarystructureimpartsthetensilestrengthofsteeltocollagen(has unusualstrength) ❖Types arrangementofcollagenfibril: a. Parallelbundles:in tendons,cartilage b. Sheets:layered atmanyanglesin skin
- 4. Arrangements of collagen fibers in cartilage of bone
- 5. Types of Collagen ❖19 different Types of Collagen , composed of 30 distinct polypeptide chains encoded by separate genes. ❖ Numbering for Types of Collagen: Roman numerals I, II, III….XIX ❖Structure of collagen types : in principle , all types of collagen are triple helical structures . The triple helix may occur throughout the molecule or only a part of the molecule. ❖Each one suited to performed specialized function in tissue ❖e.g. Collagen Type I →skin, Collagen Type II → bone
- 6. Structure of collagen Type 1 ❖ Structure of collagen Type 1: 1. Triple stranded helical structure present throughout the collagen molecule 2. Shape : rod-like molecule → 1.4 nm diameter and 300 nm length 3. Number of Amino acid residues : 1000 per for each polypeptide chain (3000 /molecule) 4. Amino acid contribution : 1/3 rd of amino acids are Glycine (every third amino acid in collagen is Glycine. 5. Repetitive amino acid sequence : (Gly – X –Y )n ,where X and Y represent other amino acids 6. Proline and hydroxyproline : 100 per for each polypeptide chain 7. Function of Proline and hydroxyproline : confer rigidity to the collagen molecule 8. Collagen Fibril formation : Triple helical molecule of collagen assemble to form elongated fibrils . It occurs by a quarter staggered alignment i.e. each triple helix is displaced longitudinally from its neighbor collagen molecule by about one-quarter of its length 9. Collagen Fiber formation : Collagen Fibrils assemble to form rod like fibers . 10. Strength of Collagen Fiber : contributed by covalent cross linking of formed between Lysine and hydroxylysine and also between Proline and hydroxyproline.
- 7. Collagen molecules in Collagen fibers Triple helical molecule of collagen assemble to form elongated fibrils . Triple stranded helical structure present throughout the collagen molecule Collagen Fibrils assemble to form rod like fibers . Repetitive amino acid sequence (Gly – X –Y )n Proline and hydroxyproline confer rigidity to the collagen molecule
- 8. Arrangement of Tropocollagen molecules in collagen fibril Heads of Tropocollagen molecules 64 nm Cross striations Sections of Tropocollagen moleculeCollagen Fibril formation : Triple helical molecule of collagen assemble to form elongated fibrils . It occurs by a quarter staggered alignment i.e. each triple helix is displaced longitudinally from its neighbor collagen molecule by about one-quarter of its length.
- 9. Tropocollagen molecule ❖Tropocollagen : Subunits of Collagen • Shape : rod shaped • Length : 300nm • Thickness : 1.4 nm • Molecular weight : 300,000 • Constituent polypeptides: three helically interwind polypeptides of equal length (each with 1000 amino acid residues) • Primary structure of collagen : all 3 or two out of three chains have identical in amino acid sequence. Rich in Glycine (35%)and Alanine(11%) , Gly-Pro-X or Gly-Hpr-X or • Repetitive amino acid sequence : (Gly – X –Y )n ,where X and Y represent other amino acids • Secondary structure of collagen : Each of three polypeptide chains of tropocollagen is itself -helix. Proline and hydroxyproline form bends in polypeptide chains that they are not compatible with -helix structure.
- 10. Collagen fibrils ❖Collagen fibril : • Triple helical molecules are associated into Collagen fibrils. • It consists of recurring polypeptide subunits called tropocollagen, arranged head to tail in parallel bundles . The heads of the tropocollagen molecules are staggered along the length of fibers ,accounting for the characteristic 64 nm spacing of the cross striations in most collagens . • A section of tropocollagen molecule shows the backbone of triple helix . Each of three polypeptide chains of tropocollagen is itself -helix whose pitch and spacing is determined by the rigid R group of the numerous Proline and hydroxyproline residue . • The gap between the end of one triple helix and the beginning of the next where there is the deposition of hydroxyapatite crystals in bone formation.
- 11. Constituent amino acids of triple stranded helix Structure of Collagen • ScvConstituent amino acids of Collagen % of total amino acids Glycine 33 Proline and hydroxy proline 21 Lysine and hydroxy Lysine 3 Alanine 11 Arginine 5 Cysteine and Tryptophan absent Scurvy:vitaminCdeficiency→failure ofhydroxylationofProlineandLysineleadstoreducedhydrogen bonding→weaknessofcollagen→Brittlebonedisease:mutation→replacementofcentralGlycine
- 12. Triple helical secondary structure of Collagen
- 13. Forces stabilizing Triple helical secondary structure of Collagen ❖Forces stabilizing Triple helical secondary structure of Collagen: 1. Hydrogen bonds : three left handed helices are bound together by interchain hydrogen bonds. 2. Lysinonorleucine bond: covalent cross links both within and between triple helical units further stabilize Collagen fibers. 3. Electrostatic interactions 4. Hydrophobic interactions
- 14. Covalent cross-links in Collagen fibers • Strength of Collagen Fiber : contributed by covalent cross linking formed between Lysine and hydroxylysine and also between Proline and hydroxyproline. • Covalent cross links are formed both within and between triple helical units further stabilize Collagen fibers. • The degree of covalent cross-linking in Collagen molecule increases with age . • In Elder individuals : skin, blood vessels (Collagen containing tissue) become less elastic and more stiff → health complications
- 15. Skin :Collagen containing tissue In Elder individuals , skin, blood vessels (Collagen containing tissue) become less elastic and more stiff → health complications
- 16. Collagen and calcific aortic valve stenosis(CAVS)
- 17. Biosynthesis of collagen ❖Biosynthesis of collagen: collagen is an extracellular protein but synthesized as an intracellular precursor molecule before becoming a mature collagen fibril. • Site : fibroblast ,osteoblasts in bones , chondroblasts in cartilage, odontoblasts in teeth • Cellular location : ribosomes in endoplasmic reticulum (ER) • Precursor : preprocollagen (a single polypeptide chain) with leader peptide at amino terminal 20000 MW and carboxy terminal 35000MW.Both are not present in mature collagen. • Function of preprocollagen: contains a signal peptide which directs the protein to each endoplasmic reticulum (ER) • Synthesis of procollagen : from preprocollagen in (ER) after cleavage of a signal peptide • Post transcriptional modification of procollagen : hydroxylation, glycosylation and disulfide formation . Followed by its secretion in extracellular medium by the way of Golgi complex . • Synthesis of collagen in extracellular medium : from preprocollagen after action of aminopeptidase and carboxypeptidase to remove terminal amino acids. This followed by a spontaneous assembly of polypeptide chains to form triple helical structure (with 1000 amino acids each) of collagen .
- 19. Structural modification of Collagen during its Synthesis Procollagen Tropocollagen Collagen Glycosylationloss of peptide potion from N-terminal and C-terminal Each of the 3 chains is in a left handed helix with 3 amino acids per turn. 3 Chains are further twisted in right handed way to give cable like structure. Hydroxylation of Proline and Lysine by Lysyl hydroxylase and Proline hydroxylase in presence of vitamin C→ Cross linking of hydroxy proline and hydroxy lysine Since vitamin C is required for collagen synthesis ,a connective tissue , there is a delay in wound healing process in vitamin C deficiency.
- 20. Functions of Collagen ❖Functions of Collagen : triple helical molecules are associated into fibrils. There is gap between the end of one triple helix and the beginning of the next where there is deposition of hydroxyapatite crystals in bone formation. 1. Gives tensile strength, support and shape to tissue . To break a collagen fiber of 1 mm in diameter, a load of 10-40 kg is needed. In disease status tensile strength is reduced. 2. Contributes to proper alignment of cells ,which in turn help in cell proliferation and their differentiation to different tissue and organs . 3. Collagen which is exposed in blood vessels contributes to thrombus formation. ❖Collagen can be converted to a. gelatin by boiling by splitting off some amino acids .Gelatin is highly soluble and easily digestible. It forms gel on cooling and is provided as diet for convalescents and invalids. But it lacks essential amino acid Tryptophan. b. a tough hard substance on treatment with tannic acid (tannic process)
- 21. Genetic aspects of Collagen Synthesis ❖Genetic aspects of Collagen Synthesis : 1. Complex process 2. Involves at least 30 genes in human 3. about 8 post –transcriptional modifications 4. Inherited diseases due to gene mutations linked with collagen synthesis: a. Ehlers-Danlos syndrome b. Alport syndrome c. Osteogenesis imperfecta d. Epidermolysis bullosa
- 22. Abnormalities associated with collagen synthesis Disease Abnormalities associated with collagen synthesis Ehlers-Danlos syndrome Inherited disorders characterized by hyperextensibility of skin and abnormal tissue fragility Alport syndrome Defect in formation of type IV collagen fibers found in the basement membrane of renal glomeruli→ hematuria and renal disease Osteogenesis imperfecta Characterized by abnormal bone fragility due to deceased synthesis of collagen Epidermolysis bullosa due to alteration in in the structure of type VII collagen fibers→ skin breaks and blister formation even with minor trauma Scurvy Deficiency of vitamin C→ defective post translational modification of collagen→ bleeding gums ,poor wound healing, subcutaneous hemorrhage Lathyrism (disease of bone deformities ) CausedbyconsumptionofLathyrussativa(kesaridal)containingtoxiccompound BetaOxalylAminoAlanine(BOAA).BOAAinhibitsenzymeLysyloxidaseand interfereswiththecrosslinkingoflysineaminoacidresiduesincollagen.
- 23. Types of Ehlers-Danlos syndrome ❖Types of Ehlers Danlos syndrome : • Ehlers-Danlos syndrome type V: inherited deficiency of Lysyl oxidase(copper requiring enzymes)→prevents cross-linking of collagen→ arterio-vascular and skeletal changes. • Ehlers-Danlos syndrome type VI: inherited deficiency of Lysyl hydroxylase →abnormalities of the eye ,severe scoliosis (abnormal vertebral curvature) and hyperextensibility of skin and joints. • Ehlers-Danlos syndrome type VII: non-serving of procollagen as a substrate for the procollagen amino protease →hip dislocation , increased skin elasticity and short stature.
- 24. Ehlers-Danlos syndrome : Clinical manifestations Hyperextensibility of skin and joints severescoliosis (abnormalvertebralcurvature) Ehlers-Danlos syndrome
- 25. Alport syndrome Alport syndrome :Defectinformationof typeIVcollagenfibersfoundinthebasementmembraneof renalglomeruli→ hematuriaandrenaldisease
- 26. Alport syndrome :Clinical manifestations Visual abnormality Deafness Glomerular Nephritis
- 28. Osteogenesis imperfecta : Clinical manifestations Osteogenesisimperfecta:Characterizedby abnormalbonefragilityduetodeceasedsynthesisofcollagen
- 29. Scurvy Scurvy: Deficiency of vitamin C→ defective post translational modification of collagen→ bleeding gums ,poor wound healing, subcutaneous hemorrhage Scurvy
- 32. Lathyrism