(17 - Salt analysis 1 to 24.pdf
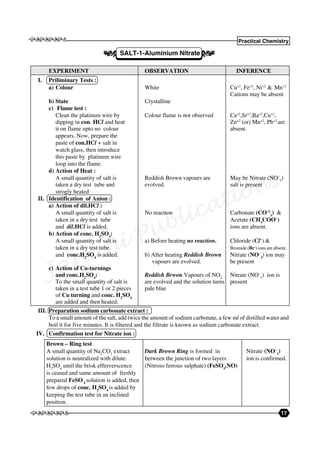
- 1. eeee 17 Practical Chemistry eeee Janani Publications EXPERIMENT OBSERVATION INFERENCE I. Priliminary Tests : : a) Colour White Cu+2 , Fe+2 , Ni+2 & Mn+2 Cations may be absent b) State Crystalline c) Flame test : Clean the platinum wire by Colour flame is not observed Ca+2 ,Sr+2 ,Ba+2 ,Cu+2 , dipping in con. HCl and heat Zn+2 (or) Mn+2 , Pb+2 are it on flame upto no colour absent. appears. Now, prepare the paste of con.HCl + salt in watch glass, then introduce this paste by platinum wire loop into the flame. d) Action of Heat : A small quantity of salt is Reddish Brown vapours are May be Nitrate (NO– 3 ) taken a dry test tube and evolved. salt is present strogly heated II. Identification of Anion : a) Action of dil.HCl : A small quantity of salt is No reaction Carbonate (CO–2 3 ) & taken in a dry test tube Acetate (CH3 COO– ) and dil.HCl is added. ions are absent. b) Action of conc. H2 SO4 : A small quantity of salt is a) Before heating no reaction. Chloride (Cl– ) & taken in a dry test tube Bromide (Br– ) ions are absent. and conc.H2 SO4 is added. b) After heating Reddish Brown Nitrate (NO– 3 ) ion may vapours are evolved. be present c) Action of Cu-turnings and conc.H2 SO4 : Reddish Brwon Vapours of NO2 Nitrate (NO– 3 ) ion is To the small quantity of salt is are evolved and the solution turns present taken in a test tube 1 or 2 pieces pale blue of Cu turning and conc. H2 SO4 are added and then heated. SALT-1-Aluminium Nitrate . . III. Preparation sodium carbonate extract : To a small amount of the salt, add twice the amount of sodium carbonate, a few ml of distilled water and boil it for five minutes. It is filtered and the filtrate is known as sodium carbonate extract. IV. Confirmation test for Nitrate ion : Brown – Ring test A small quantity of Na2 CO3 extract Dark Brown Ring is formed in Nitrate (NO– 3 ) solution is neutralized with dilute. between the junction of two layers ion is confirmed. H2 SO4 until the brisk efferverscence (Nitroso ferrous sulphate) (FeSO4 .NO) is ceased and same amount of freshly prepared FeSO4 solution is added, then few drops of conc. H2 SO4 is added by keeping the test tube in an inclined position.
- 2. Practical Chemistry eeee 18 eeee Janani Publications V. Identification of Cation : Preparation of original salt solution : Original salt solution can be prepared by dissolving excess amount of given salt in a suitable solvent. VI. Test for Ammonium (NH+ 4 )ion : Reaction with NaOH : To the small colour less gas (NH3 ) with Ammonium (NH+ 4 ) quantity of salt is taken in a dry test pungent smell is not evolved. cation is absent. tube few drops of NaOH solution is added and heated strongly. VII. Group Separation Table : a) Test for I Group Cations : To the salt solution a few drops of No ppt. is formed. Lead (Pb+2 ) cation is dil HCl solution is added. absent. b) Test for II Group Cations : To the salt solution a few drops of No ppt. is formed. Copper (Cu+2 ) cation dil HCl solution is added and is absent. H2 S gas is passed. c) Test for III Group Cations: To the salt solution NH4 Cl White gelatinous ppt. is formed. Aluminium (Al+3 ) salt and NH4 OH solution are cation is present added. d) Test for IV Group Cations: Salt solution + NH4 Cl salt + White ppt. ZnS is not formed. Zinc (Zn+2 ) absent NH4 OH solution + H2 S gas passed Flesh colour ppt. MnS is not formed. Manganese (Mn+2 ) absent Black ppt. NiS is not formed. Nickel (Ni+2 ) absent e) Test for V Group Cations: Salt solution + NH4 Cl salt + White ppt. CaCO3 is not formed. Calcium (Ca+2 ) absent NH4 OH solution +(NH4 )2 CO3 White ppt. BaCO3 is not formed. Barium (Ba+2 ) absent solution is added White ppt. SrCO3 is not formed. Strontium (Sr+2 ) absent f) Test for VI Group Cations : Salt solution + NH4 Cl salt + White ppt. is not formed Magnesium (Mg+2 ) NH4 OH solution +(NH4 )2 CO3 + absent Na2 HPO4 solution is added VIII. Confirmation Tests for Aluminium : a) Actions of NaOH solution: To the salt solution NaOH White gelatinous ppt. is formed. Aluminium (Al+3 ) solution is added. which is soluble in excess of cation is confirmed. NaOH solution b) Action of NH4 OH solution: To the salt solution NH4 OH White gelatinous ppt. is formed. Aluminium (Al+3 ) solution is added. which is soluble in excess of cation is confirmed. NH4 OH solution. IX. Report : The given Cation is : Aluminium (Al+3 ) ; The given Anion is : Nitrate (NO– 3 ) The given salt is : Aluminium Nitrate [Al (NO3 )3 ]
- 3. eeee 19 Practical Chemistry eeee Janani Publications EXPERIMENT OBSERVATION INFERENCE I. Priliminary Tests : : a) Colour : White Cu+2 , Fe+2 , Ni+2 & Mn+2 Cations may be absent. b) State Crystalline c) Flame test : Clean the platinum wire by Colour flame is not observed Ca+2 , Sr+2 ,Ba+2 , Cu+2 , Zn+2 dipping in con.HCl and (or) Mn+2 , Pb+2 are absent. heat it on flame upto no colour appears. Now,prepare the paste of con.HCl + salt in watch glass, then introduce this paste by platinum wire loop into the flame. d) Action of heat : A small quantity of salt is Water droplets are formed on May be Hydrated salt taken in a dry test tube and the inner walls of test tube strongly heated II. Identification of Anion : a) Action of dil.HCl : A small quantity of salt is No reaction Carbonate (CO–2 3 ) & taken in a dry test tube Acetate (CH3 COO– ) ions and dil.HCl is added are absent. b) Action of conc.H2 SO4 : A small quntity of salt is a) Before heating no reaction Chloride (Cl– ) & taken in a dry test tube bromide (Br– ) ions are absent. and conc. H2 SO4 is added b) After heating no reaction. Nitrate (NO– 3 )ion is absent c) Action of Cu-turnings and conc. H2 SO4 : To the small quantity of No reaction Nitrate (NO– 3 ) ion is absent. salt is a taken in test tube 1 or 2 pieces of Cu turnings and conc. H2 SO4 are added and then heated. d) Action of BaCl2 solution : To the salt solution BaCl2 White ppt. of (BaSO4 ) Sulphate (SO–2 4 ) ion is solution is added. is formed. present III. Preparation sodium carbonate extract : To a small amount of the salt, add twice the amount of sodium carbonate, a few ml of distilled water and boil it for five minutes. It is filtered and the filtrate is known as sodium carbonate extract. IV. Confirmation test for Sulphate ion : Action of BaCl2 solution : To the salt solution BaCl2 White ppt. of (BaSO4 ) is formed. Sulphate (SO–2 4 ) ion is solution is added. Solubility : Above ppt. is insoluble confirmed. in Conc. HCl SALT-2-Aluminium Sulphate . .
- 4. Practical Chemistry eeee 20 eeee Janani Publications V. Identification of Cation : Preparation of original salt solution : Original salt solution can be prepared by dissolving excess amount of given salt in a suitable solvent. VI. Test for Ammonium (NH+ 4 )ion : Reaction with NaOH : To the small colour less gas (NH3 ) with Ammonium (NH+ 4 ) quantity of salt is taken in a dry test pungent smell is not evolved. cation is absent. tube few drops of NaOH solution is added and heated strongly. VII.Group Separation Table : a) Test for I Group Cations : To the salt solution a few drops of No ppt. is formed. Lead (Pb+2 ) cation is dil HCl solution is added. absent. b) Test for II Group Cations : To the salt solution a few drops of No ppt. is formed. Copper (Cu+2 ) cation dil HCl solution is added and is absent. H2 S gas is passed. c) Test for III Group Cations: To the salt solution NH4 Cl White gelatinous ppt. is formed. Aluminium (Al+3 ) salt and NH4 OH solution are cation is present added. d) Test for IV Group Cations: Salt solution + NH4 Cl salt + White ppt. ZnS is not formed. Zinc (Zn+2 ) absent NH4 OH solution + H2 S gas passed Flesh colour ppt. MnS is not formed. Manganese (Mn+2 ) absent Black ppt. NiS is not formed. Nickel (Ni+2 ) absent e) Test for V Group Cations: Salt solution + NH4 Cl salt + White ppt. CaCO3 is not formed. Calcium (Ca+2 ) absent NH4 OH solution +(NH4 )2 CO3 White ppt. BaCO3 is not formed. Barium (Ba+2 ) absent solution is added White ppt. SrCO3 is not formed. Strontium (Sr+2 ) absent f) Test for VI Group Cations : Salt solution + NH4 Cl salt + White ppt. is not formed Magnesium (Mg+2 ) NH4 OH solution +(NH4 )2 CO3 + absent Na2 HPO4 solution is added VIII. Confirmation Tests for Aluminium : a) Action of NaOH Solution : To the salt solution NaOH White gelatinous ppt. is formed. Aluminium (Al+3 ) solution is added Which is soluble in excess of Cation is confirmed. NaOH Solution. b) Action of NH4 OH solution : To the salt solution NH4 OH White gelatinous ppt. is formed. Aluminium (Al+3 ) solution is added. Which is soluble in excess of cation is confirmed. NH4 OH solution. IX. Report : The given Cation is : Aluminium (Al+3 ) The given Anion is : Sulphate (SO–2 4 ) The given salt is : Aluminium Sulphate [Al2 (SO4 )3 ]
- 5. eeee 21 Practical Chemistry eeee Janani Publications EXPERIMENT OBSERVATION INFERENCE I. Priliminary Tests : a) Colour : White Cu+2 , Fe+2 , Ni+2 & Mn+2 b) State Crystalline Cations may be absent. c) Flame test : Clean the platinum wire by Colour flame is not observed Ca+2 , Sr+2 ,Ba+2 ,Cu+2 , Zn+2 dipping in con.HCl and heat (or) Mn+2 , Pb+2 are absent it on flame upto no colour appears. Now, prepare the paste of con.HCl + salt in watch glass, then introduce this paste by platinum wire loop into the flame. d) Action of heat : A small quantity of salt is Charateristics smell of vinegar May be Acetate taken in a dry test tube and (CH3 COO– ) ion is present. strongly heated. II. Identification of Anion : a) Action of dil.HCl : A small quantity of salt is Colourless vapours with smell May be Acetate (CH3 COO– ) taken in a dry test tube and of vinegar is evolved and ion is present dil.HCl is added. which turns blue litmus to red. III. Preparation sodium carbonate extract : To a small amount of the salt, add twice the amount of sodium carbonate, a few ml of distilled water and boil it for five minutes. It is filtered and the filtrate is known as sodium carbonate extract. IV. Confirmation test for Acetate ion : a) Action of Neutral FeCl3 solution : To the small quantity of salt Deep Red filtrate is formed. Acetate (CH3 COO– ) is taken in a test tube and On boiling above solution with ion is confirmed. neutral FeCl3 solution is water is becomes brownish red ppt. added. b) Esterification test : To the salt solution Fruity odour is obtained. Acetate (CH3 COO – ) conc. H2 SO4 and C2 H5 OH ion is confirmed. are added and heated. It poured into water SALT-3-Ammonium Acetate . . V. Identification of Cation : Preparation of original salt solution : Original salt solution can be prepared by dissolving excess amount of given salt in a suitable solvent.
- 6. Practical Chemistry eeee 22 eeee Janani Publications VI. Test for Ammonium (NH+ 4 )ion : Reaction with NaOH : To the small quantity of salt is colour less gas with pungent Ammonium (NH4 + ) taken in a dry test tube a few smell of ammonia (NH3 ) is cation may be present. drops of NaOH solution is evolved and gives dense white added and heated strongly. fumes (NH4 Cl) with glass rod when dipped in conc.HCl Note: If Ammonium cation is identified then write following table. Do confirmation test of Ammonium Cation. Group Group Reagents Name of the Precipitate Name of the Cation (Identification Tests of cations) a) Salt solution + dil. HCl is added White ppt. PbCl2 is not formed. Lead (Pb+2 ) absent b) Salt solution + dil.HCl + H2 S gas passed Black ppt. CuS is not formed. Copper (Cu+2 ) absent c) Salt solution + NH4 Cl salt + White gelatinous ppt.Al(OH)3 is Aluminium (Al+3 ) absent NH4 OH solution is added not formed. Dirty Green ppt. Fe (OH)2 is not Ferrous (Fe+2 ) absent formed. e) Salt solution + NH4 Cl salt + White ppt. ZnS is not formed. Zinc (Zn+2 ) absent NH4 OH solution + H2 S gas Flesh colour ppt. MnS is not Manganese (Mn+2 ) absent passed formed. Black ppt. NiS is not formed. Nickel (Ni+2 ) absent f) Salt solution + NH4 Cl salt + White ppt. CaCO3 is not formed. Calcium (Ca+2 ) absent NH4 OH solution +(NH4 )2 CO3 White ppt. BaCO3 is not formed. Barium (Ba+2 ) absent solution is added White ppt. SrCO3 is not formed. Strontium (Sr+2 ) absent g) Salt solution + NH4 Cl salt + White ppt. is not formed Magnesium (Mg+2 ) NH4 OH solution +(NH4 )CO3 + absent Na2 HPO4 solution is added VII. Confirmation Tests for Ammonium : Action of Nessler’s reagent : To the salt solution Nessler’s Reddish Brown colour ppt. is . Ammonium (NH+ 4 ) reagent [K2 HgI4 ] is added. formed (Iodide of Million’s base) cation is confirmed VIII. Report : The given Cation is :Ammonium (NH+ 4 ) The given Anion is : Acetate (CH3 COO– ) The given salt is : Ammonium Acetate [NH4 (CH3 COO)]
- 7. eeee 23 Practical Chemistry eeee Janani Publications EXPERIMENT OBSERVATION INFERENCE I. Priliminary Tests : a) Colour : White Cu+2 , Fe+2 , Ni+2 & Mn+2 b) State : Crystalline Cations may be absent. c) Flame test : Clean the platinum wire by Colour flame is not observed Ca+2 , Sr+2 ,Ba+2 ,Cu+2 , Zn+2 dipping in con.HCl and heat (or) Mn+2 , Pb+2 are absent it on flame upto no colour appears. Now, prepare the paste of con.HCl + salt in watch glass, then introduce this paste by platinum wire loop into the flame. d) Action of heat : A small quantity of salt is Colourless gas with ammonia May be Ammonium taken in a dry test tube and smell is evolved sublimate (NH+ 4 ) salt is present. strongly heated is formed II. Identification of Anion : a) Action of dil.HCl : A small quantity of salt No reaction Carbonate (CO3 –2 ) & is taken in a dry test tube Acetate (CH3 COO– ) and dil.HCl is added. ions are absent. b) Action of Conc. H2 SO4 : A small quantity of salt is Reddish Brown vapours with Bromide (Br– ) ion taken in a dry test tube and pungent smell are evolved. may be confirmed. Conc. H2 SO4 is added. Which turns starch to Yellow. III. Preparation sodium carbonate extract : To a small amount of the salt, add twice the amount of sodium carbonate, a few ml of distilled water and boil it for five minutes. It is filtered and the filtrate is known as sodium carbonate extract. IV. Confirmation test for Bromide ion : a) Action of MnO2 & Conc. H2 SO4 : To the small quantity of salt is Reddish Brown vapour (Br2 ) Bromide (Br– ) ion is taken in a test tube pinch of with pungent smell are evolved. confirmed. MnO2 and conc. H2 SO4 are Which turns starch to yellow. added. b) Action of AgNO3 Solution : To the salt solution AgNO3 Pale yellow ppt. of (AgBr) is Bromide (Br– ) ion solution is added is formed. confirmed Solubility : Above ppt. is partially soluble in NH4 OH solution SALT-4-Ammonium Bromide . .
- 8. Practical Chemistry eeee 24 eeee Janani Publications V. Identification of Cation : Preparation of original salt solution : Original salt solution can be prepared by dissolving excess amount of given salt in a suitable solvent. VI. Test for Ammonium (NH+ 4 )ion : Reaction with NaOH : To the small quantity of salt is colour less gas with pungent Ammonium (NH4 + ) taken in a dry test tube a few smell of ammonia (NH3 ) is cation may be present. drops of NaOH solution is evolved and gives dense white added and heated strongly. fumes (NH4 Cl) with glass rod when dipped in conc.HCl Note: If Ammonium cation is identified then write following table. Do confirmation test of Ammonium Cation. Group Group Reagents Name of the Precipitate Name of the Cation (Identification Tests of cations) a) Salt solution + dil. HCl is added White ppt. PbCl2 is not formed. Lead (Pb+2 ) absent b) Salt solution + dil.HCl + H2 S gas passed Black ppt. CuS is not formed. Copper (Cu+2 ) absent c) Salt solution + NH4 Cl salt + White gelatinous ppt.Al(OH)3 is Aluminium (Al+3 ) absent NH4 OH solution is added not formed. Dirty Green ppt. Fe (OH)2 is not Ferrous (Fe+2 ) absent formed. e) Salt solution + NH4 Cl salt + White ppt. ZnS is not formed. Zinc (Zn+2 ) absent NH4 OH solution + H2 S gas Flesh colour ppt. MnS is not Manganese (Mn+2 ) absent passed formed. Black ppt. NiS is not formed. Nickel (Ni+2 ) absent f) Salt solution + NH4 Cl salt + White ppt. CaCO3 is not formed. Calcium (Ca+2 ) absent NH4 OH solution +(NH4 )2 CO3 White ppt. BaCO3 is not formed. Barium (Ba+2 ) absent solution is added White ppt. SrCO3 is not formed. Strontium (Sr+2 ) absent g) Salt solution + NH4 Cl salt + White ppt. is not formed Magnesium (Mg+2 ) NH4 OH solution +(NH4 )CO3 + absent Na2 HPO4 solution is added VII. Confirmation Tests for Ammonium : Action of Nessler’s reagent : To the salt solution Nessler’s Reddish Brown colour ppt. is . Ammonium (NH+ 4 ) reagent [K2 HgI4 ] is added. formed (Iodide of Million’s base) cation is confirmed VIII. Report : The given Cation is :Ammonium (NH+ 4 ) The given Anion is : Bromide (Br– ) The given salt is : Ammonium Bromide [NH4 Br]
- 9. eeee 25 Practical Chemistry eeee Janani Publications EXPERIMENT OBSERVATION INFERENCE I. Priliminary Tests : a) Colour : White Cu+2 , Fe+2 , Ni+2 & Mn+2 Cations may be absent. b) State : Crystalline c) Flame test : Clean the platinum wire by Colour flame is Ca+2 , Sr+2 , Ba+2 , Cu+2 , dipping in con.HCl and heat not observed Zn+2 (or) Mn+2 , Pb+2 are it on flame upto no colour absent appears. Now, prepare the paste of con.HCl + salt in watch glass, then introduce this paste by platinum wire loop into the flame. d) Action of heat : A small quantity of salt is Colourless gas with May be Ammonium taken in a dry test tube and ammonia smell is evolved. (NH+ 4 ) salt is present. strongly heated. II. Identification of Anion : a) Action of dil.HCl : A small quantity of salt is Colour and smell less gas Carbonate (CO–2 3 ) ion taken in a dry test tube and (CO2 ) evolved with brisk may be present dil.HCl is added. effervescence. The gas puts off a burning splinter. It turns Lime water into milky. III. Preparation sodium carbonate extract : To a small amount of the salt, add twice the amount of sodium carbonate, a few ml of distilled water and boil it for five minutes. It is filtered and the filtrate is known as sodium carbonate extract. IV. Confirmation test for Carbonate ion : Action of BaCl2 solution : A small quantity of salt solution White ppt. (BaCO3 ) is formed. Carbonate (CO–2 3 ) ion is taken in a dry test tube and Solubility : Above ppt. is soluble is confirmed. BaCl2 solution is added dil.HCl V. Identification of Cation Preparation of original salt solution : Original salt solution can be prepared by dissolving excess amount of given salt in a suitable solvent. SALT-5-Ammonium Carbonate . .
- 10. Practical Chemistry eeee 26 eeee Janani Publications VI. Test for Ammonium (NH+ 4 )ion : Reaction with NaOH : To the small quantity of salt is Colour less gas with pungent Ammonium (NH+ 4 ) cation taken in a dry test tube a few smell of ammonia (NH3 ) is may be present drops of NaOH solution is evolved and gives dense white added and heated strongly. fumes (NH4 Cl) with glass rod when dipped in conc.HCl Note : If Ammonium cation is identified then write following table. Do confirmation test of Ammonium cation Group Group Reagents Name of the Precipitate Name of the Cation (Identification Tests of cations) a) Salt solution + dil. HCl is added White ppt. PbCl2 is not formed. Lead (Pb+2 ) absent b) Salt solution + dil.HCl + H2 S gas passed Black ppt. CuS is not formed. Copper (Cu+2 ) absent c) Salt solution + NH4 Cl salt + White gelatinous ppt.Al(OH)3 is Aluminium (Al+3 ) absent NH4 OH solution is added not formed. Dirty Green ppt. Fe (OH)2 is not Ferrous (Fe+2 ) absent formed. e) Salt solution + NH4 Cl salt + White ppt. ZnS is not formed. Zinc (Zn+2 ) absent NH4 OH solution + H2 S gas Flesh colour ppt. MnS is not Manganese (Mn+2 ) absent passed formed. Black ppt. NiS is not formed. Nickel (Ni+2 ) absent f) Salt solution + NH4 Cl salt + White ppt. CaCO3 is not formed. Calcium (Ca+2 ) absent NH4 OH solution +(NH4 )2 CO3 White ppt. BaCO3 is not formed. Barium (Ba+2 ) absent solution is added White ppt. SrCO3 is not formed. Strontium (Sr+2 ) absent g) Salt solution + NH4 Cl salt + White ppt. is not formed Magnesium (Mg+2 ) NH4 OH solution +(NH4 )CO3 + absent Na2 HPO4 solution is added VII. Confirmation Tests for Ammonium : Action of Nessler’s reagent : To the salt solution Nessler’s Reddish Brown colour ppt. is Ammonium (NH+ 4 ) agent [K2 HgI4 ] is added. formed (Iodide of Million’s base) cation is confirmed VIII. Report : The given Cation is : Ammonium (NH+ 4 ) The given Anion is : Carbonate (CO–2 3 ) The given salt is : Ammonium Carbonate [ (NH4 )2 CO3 ]
- 11. eeee 27 Practical Chemistry eeee Janani Publications EXPERIMENT OBSERVATION INFERENCE I. Priliminary Tests : : a) Colour : White Cu+2 , Fe+2 , Ni+2 & Mn+2 Cations may be absent b) State : Crystalline c) Flame test : Clean the platinum wire by Colour flame is not observed Ca+2 , Sr+2 ,Ba+2 ,Cu+2 , Zn+2 dipping in con. HCl and heat it (or) Mn+2 , Pb+2 are absent on flame upto no colour appears. Now, prepare the paste of con.HCl + salt in watch glass, then introduce this paste by platinum wire loop into the flame. d) Action of heat : A small quantity of salt is taken Colourless gas with ammonia May be Ammonium in a dry test tube and strongly smell is evolved and white (NH+ 4 ) salt is present. heated. sublimate is formed. II. Identification of Anion : a) Action of dil.HCl : A small quantity of salt is No reaction Carbonate (CO–2 3 ) & taken in a dry test tube and Acetate (CH3 COO– ) dil.HCl is added. ions are absent b) Action of Conc. H2 SO4 : A small quntity of salt is Colour less gas (HCl) with Chloride (Cl– ) ion taken in a dry test tube and pungent small is evolved and may be present conc.H2 SO4 are added. gives dense white fumes (NH4 Cl) with glass rod when dipped in NH4 OH III. Preparation sodium carbonate extract : To a small amount of the salt, add twice the amount of sodium carbonate, a few ml of distilled water and boil it for five minutes. It is filtered and the filtrate is known as sodium carbonate extract. IV. Confirmation test for Carbonate ion : a)Action of MnO2 & Conc.H2 SO4 : To the small quantity of salt Greenish yellow colour Chloride (Cl– ) ion is is taken in a test tube pinch of vapours (Cl2 ) with pungent smell confirmed. MnO2 and Conc.H2 SO4 are evolved. are added. b)Action of AgNO3 solution : To the salt solution AgNO3 White curdy ppt. of (AgCl) is Chloride (Cl– ) ion solution is added. formed. is confirmed. Solubility: Above ppt. is completely soluble in NH4 OH SALT-6-Ammonium Chloride . .
- 12. Practical Chemistry eeee 28 eeee Janani Publications V. Identification of Cation : Preparation of original salt solution : Original salt solution can be prepared by dissolving excess amount of given salt in a suitable solvent. VI. Test for Ammonium (NH+ 4 )ion : Reaction with NaOH : To the small quantity of salt is Colour less gas with pungent Ammonium (NH+ 4 ) cation taken in a dry test tube a few smell of ammonia (NH3 ) is may be present drops of NaOH solution is evolved and gives dense white added and heated strongly. fumes (NH4 Cl) with glass rod when dipped in conc.HCl Note : If Ammonium cation is identified then write following table. Do confirmation test of Ammonium cation Group Group Reagents Name of the Precipitate Name of the Cation (Identification Tests of cations) a) Salt solution + dil. HCl is added White ppt. PbCl2 is not formed. Lead (Pb+2 ) absent b) Salt solution + dil.HCl + H2 S gas passed Black ppt. CuS is not formed. Copper (Cu+2 ) absent c) Salt solution + NH4 Cl salt + White gelatinous ppt.Al(OH)3 is Aluminium (Al+3 ) absent NH4 OH solution is added not formed. Dirty Green ppt. Fe (OH)2 is not Ferrous (Fe+2 ) absent formed. e) Salt solution + NH4 Cl salt + White ppt. ZnS is not formed. Zinc (Zn+2 ) absent NH4 OH solution + H2 S gas Flesh colour ppt. MnS is not Manganese (Mn+2 ) absent passed formed. Black ppt. NiS is not formed. Nickel (Ni+2 ) absent f) Salt solution + NH4 Cl salt + White ppt. CaCO3 is not formed. Calcium (Ca+2 ) absent NH4 OH solution +(NH4 )2 CO3 White ppt. BaCO3 is not formed. Barium (Ba+2 ) absent solution is added White ppt. SrCO3 is not formed. Strontium (Sr+2 ) absent g) Salt solution + NH4 Cl salt + White ppt. is not formed Magnesium (Mg+2 ) NH4 OH solution +(NH4 )CO3 + absent Na2 HPO4 solution is added VII. Confirmation Tests for Ammonium : Action of Nessler’s reagent : To the salt solution Nessler’s Reddish Brown colour ppt. is. Ammonium (NH+ 4 ) reagent [K2 HgI4 ] is added. formed (Iodide of Million’s base) cation is confirmed VIII. Report : The given Cation is : Ammonium (NH+ 4 ) The given Anion is : Chloride (Cl– ) The given salt is : Ammonium Chloride [NH4 Cl]
- 13. eeee 29 Practical Chemistry eeee Janani Publications EXPERIMENT OBSERVATION INFERENCE I. Priliminary Tests : : a) Colour : White Cu+2 , Fe+2 , Ni+2 & Mn+2 Cations may be absent b) State : Crystalline c) Flame test : Clean the platinum wire by Colour flame is not observed Ca+2 , Sr+2 , Ba+2 , Cu+2 , Zn+2 dipping in con. HCl and heat it (or) Mn+2 , Pb+2 are absent on flame upto no colour appears. Now, prepare the paste of con.HCl + salt in watch glass, then introduce this paste by platinum wire loop into the flame. d) Action of heat : A small quantity of salt is taken Colourless gas with ammonia May be Ammonium in a dry test tube and strongly smell is evolved (NH+ 4 ) salt is present. heated. II. Identification of Anion : a) Action of dil.HCl : A small quantity of salt No reaction Carbonate (CO–2 3 ) & is taken in a dry test tube and Acetate (CH3 COO– ) dil.HCl is added. ions are absent b) Action of Conc. H2 SO4 : A small quntity of salt is taken a) Before heating no reaction. Chloride (Cl– ) &Bromide in a dry test tube and b) After heating no reaction. (Br– ) ion are absent Conc. H2 SO4 is added Nitrate (NO– 3 ) ion is absent. c) Action of Cu turnings and Conc. H2 SO4 : To the small quantity of salt No reaction Nitrate (NO– 3 ) ion is absent. is taken in a test tube 1 or 2 pieces of Cu turnings and Conc.H2 SO4 are added and then heated. d) Action of BaCl2 solution: To the salt solution BaCl2 White ppt. of (BaSO4 ) is Sulphate (SO-2 4 ) ion is solution is added. formed. present. SALT-7-Ammonium Sulphate . . III. Preparation sodium carbonate extract : To a small amount of the salt, add twice the amount of sodium carbonate, a few ml of distilled water and boil it for five minutes. It is filtered and the filtrate is known as sodium carbonate extract.
- 14. Practical Chemistry eeee 30 eeee Janani Publications IV. Confirmation test for Sulphate ion : Action of BaCl2 Solution: To the salt solution BaCl2 White ppt. of (BaSO4 ) is formed. Sulphate (SO–2 4 ) ion is solution is added. Solubility : Above ppt. is insoluble confirmed. in Conc. HCl V. Identification of Cation : Preparation of original salt solution : Original salt solution can be prepared by dissolving excess amount of given salt in a suitable solvent. VI. Test for Ammonium (NH+ 4 )ion : Reaction with NaOH : To the small quantity of salt is Colour less gas with pungent Ammonium (NH+ 4 ) cation taken in a dry test tube a few smell of ammonia (NH3 ) is may be present drops of NaOH solution is evolved and gives dense white added and heated strongly. fumes (NH4 Cl) with glass rod when dipped in conc.HCl Note : If Ammonium cation is dentified then write following table. Do confirmation test of Ammonium cation Group Group Reagents Name of the Precipitate Name of the Cation (Identification Tests of cations) a) Salt solution + dil. HCl is added White ppt. PbCl2 is not formed. Lead (Pb+2 ) absent b) Salt solution + dil.HCl + H2 S gas passed Black ppt. CuS is not formed. Copper (Cu+2 ) absent c) Salt solution + NH4 Cl salt + White gelatinous ppt.Al(OH)3 is Aluminium (Al+3 ) absent NH4 OH solution is added not formed. Dirty Green ppt. Fe (OH)2 is not Ferrous (Fe+2 ) absent formed. e) Salt solution + NH4 Cl salt + White ppt. ZnS is not formed. Zinc (Zn+2 ) absent NH4 OH solution + H2 S gas Flesh colour ppt. MnS is not Manganese (Mn+2 ) absent passed formed. Black ppt. NiS is not formed. Nickel (Ni+2 ) absent f) Salt solution + NH4 Cl salt + White ppt. CaCO3 is not formed. Calcium (Ca+2 ) absent NH4 OH solution +(NH4 )2 CO3 White ppt. BaCO3 is not formed. Barium (Ba+2 ) absent solution is added White ppt. SrCO3 is not formed. Strontium (Sr+2 ) absent g) Salt solution + NH4 Cl salt + White ppt. is not formed Magnesium (Mg+2 ) NH4 OH solution +(NH4 )CO3 + absent Na2 HPO4 solution is added VII. Confirmation Tests for Ammonium : Action of Nessler’s reagent : To the salt solution Nessler’s Reddish Brown colour ppt. is. Ammonium (NH+ 4 ) reagent [K2 HgI4 ] is added. formed (Iodide of Million’s base) cation is confirmed VIII. Report : The given Cation is : Ammonium (NH+ 4 ) The given Anion is : Sulphate (SO4 –2 ) The given salt is : Ammonium Sulphate [(NH4 )2 SO4 ]
- 15. eeee 31 Practical Chemistry eeee Janani Publications EXPERIMENT OBSERVATION INFERENCE I. Priliminary Tests : : a) Colour : White Cu+2 , Fe+2 , Ni+2 & Mn+2 Cations may be absent b) State : Crystalline c) Flame test : Clean the platinum wire by Grassy - green colour flame Ba+2 May be present dipping in con.HCl and heat it is observed on flame upto no colour appears. Now, prepare the paste of con.HCl + salt in watch glass, then introduce this paste by platinum wire loop into the flame. d) Action of heat : A small quantity of salt is taken in Charateristic smell of vinegar May be acetate a dry test tube and strongly (CH3 COO– ) ion is present heated. II. Identification of Anion : a) Action of dil.HCl : A small quantity of salt is Colourless vapours with May be Acetate taken in a dry test tube and smell of vinegar is evolved (CH3 COO– ) dil.HCl is added. and which turns blue litmus ions are present to red III. Preparation sodium carbonate extract : To a small amount of the salt, add twice the amount of sodium carbonate, a few ml of distilled water and boil it for five minutes. It is filtered and the filtrate is known as sodium carbonate extract. IV. Confirmation test for Acetate ion : a) Action of Neutral FeCl3 solution : To the small quantity of salt Deep Red filtrate is formed. Acetate (CH3 COO– ) ion is is taken in a test tube and On boiling above solution with confirmed. neutral FeCl3 solution is water it becomes brownish added red ppt b) Esterification test : To the salt solution Fruity odour is obtained. Acetate (CH3 COO– ) ion is Conc. H2 SO4 and C2 H5 OH confirmed. are added and heated. It is poured into water V. Identification of Cation : Preparation of original salt solution : Original salt solution can be prepared by dissolving excess amount of given salt in a suitable solvent. SALT-8-Barium Acetate . .
- 16. Practical Chemistry eeee 32 eeee Janani Publications V. Identification of Cation : Preparation of original salt solution : Original salt solution can be prepared by dissolving excess amount of given salt in a suitable solvent. VI. Test for Ammonium (NH+ 4 )ion : Reaction with NaOH : To the small quantity of slat is colour less gas (NH3 ) with pungent Ammonium (NH+ 4 ) taken in a dry test tube a few smell is not evolved. cation is absent. drops of NaOH solution is added and strongly heated VII.Group Separation Table : a) Test for I Group Cations : To the salt solution a few drops of No ppt. is formed. Lead (Pb+2 ) cation is dil HCl solution is added. absent. b) Test for II Group Cations : To the salt solution a few drops of No ppt. is formed. Copper (Cu+2 ) cation dil HCl solution is added and is absent. H2 S gas is passed. c) Test for III Group Cations: To the salt solution NH4 Cl No ppt. is formed. Aluminium (Al+3 ) salt and NH4 OH solution are cation absent added. d) Test for IV Group Cations: Salt solution + NH4 Cl salt + White ppt. ZnS is not formed. Zinc (Zn+2 ) absent NH4 OH solution + H2 S gas passed Flesh colour ppt. MnS is not formed. Manganese (Mn+2 ) absent Black ppt. NiS is not formed. Nickel (Ni+2 ) absent e) Test for V Group Cations : Salt solution + NH4 Cl salt + White ppt. is formed Calcium (Ca+2 ) NH4 OH solution +(NH4 )2 CO3 Barium (Ba+2 ) solution is added Strontium (Sr+2 ) cations are present f) Test for VI Group Cations : Salt solution + NH4 Cl salt + White ppt. is not formed Magnesium (Mg+2 ) NH4 OH solution +(NH4 )2 CO3 + absent Na2 HPO4 solution is added VIII. Confirmation Tests for Barium : a) Action of K2 CrO4 solution : To the salt solution potassium Yellow colour ppt. (BaCrO4 ) is Barium (Ba+2 ) cation chromate solution (K2 CrO4 ) is formed which is soluble in may be present. added. dil.Acetic acid and soluble in dil HCl b) Action of Ammonium oxalate soution: To the salt solution Ammonium White colour ppt. (BaC2 O4 ) Barium (Ba+2 ) cation oxalate solution is added. is formed. Above ppt. is soluble is confirmed. in hot Acetic acid. VIII. Report : The given Cation is : Barium (Ba+2 ) ; The given Anion is : Acetate (CH3 COO– ) The given salt is : Barium Acetate [Ba(CH3 COO)2 ]
- 17. eeee 33 Practical Chemistry eeee Janani Publications EXPERIMENT OBSERVATION INFERENCE I. Priliminary Tests : : a) Colour : White Cu+2 , Fe+2 , Ni+2 & Mn+2 Cations may be absent b) State : Crystalline c) Flame test : Clean the platinum wire by Grassy-green colour flame Ba+2 May be present dipping in con.HCl and heat is observed it on flame upto no colour appears. Now, prepare the paste of con.HCl + salt in watch glass, then introduce this paste by platinum wire loop into the flame. d) Action of heat : A small quantity of salt is taken Water droplets are formed on the May be Hydrated salt. in a dry test tube and strongly inner wall of test tube. heated. II. Identification of Anion : a) Action of dil.HCl : A small quntity of Salt is taken No reaction Carbonate (CO–2 3 ) & in a dry test tube and dil. HCl Acetate (CH3 COO– ) is added. ions are absent. b) Action of conc.H2 SO4 : A small quantity of salt is taken Raddish brown vapours Bromide (Br– ) ion in a dry test tube and with pungent smell are may be present conc. H2 SO4 is added evolved . Which turns starch to yellow III. Preparation sodium carbonate extract : To a small amount of the salt, add twice the amount of sodium carbonate, a few ml of distilled water and boil it for five minutes. It is filtered and the filtrate is known as sodium carbonate extract. IV. Confirmation test for Bromide ion : a) Action of MnO2 & Conc. H2 SO4 : To the small quantity of salt is Reddish Brown vapour (Br2 ) with Bromide (Br– ) ion taken in a test tube pinch of MnO2 pungent smell are evolved. Which is confirmed. and Conc. H2 SO4 are added. turns starch to yellow. b) Action of AgNO3 solution : To the salt solution AgNO3 Pale yellow ppt. of (AgBr) is formed. Bromide (Br– ) ion solution is added. Solubility : Above ppt. is partially is confirmed. soluble in NH4 OH solution V. Identification of Cation : Preparation of original salt solution : Original salt solution can be prepared by dissolving excess amount of given salt in a suitable solvent. SALT-9-Barium Bromide . .
- 18. Practical Chemistry eeee 34 eeee Janani Publications VI. Test for Ammonium (NH+ 4 )ion : Reaction with NaOH : To the small quantity of slat is colour less gas (NH3 ) with pungent Ammonium (NH+ 4 ) taken in a dry test tube a few smell is not evolved. cation is absent. drops of NaOH VII.Group Separation Table : a) Test for I Group Cations : To the salt solution a few drops of No ppt. is formed. Lead (Pb+2 ) cation is dil HCl solution is added. absent. b) Test for II Group Cations : To the salt solution a few drops of No ppt. is formed. Copper (Cu+2 ) cation dil HCl solution is added and is absent. H2 S gas is passed. c) Test for III Group Cations: To the salt solution NH4 Cl No ppt. is formed. Aluminium (Al+3 ) salt and NH4 OH solution are cation absent added. d) Test for IV Group Cations: Salt solution + NH4 Cl salt + White ppt. ZnS is not formed. Zinc (Zn+2 ) absent NH4 OH solution + H2 S gas passed Flesh colour ppt. MnS is not formed. Manganese (Mn+2 ) absent Black ppt. NiS is not formed. Nickel (Ni+2 ) absent e) Test for V Group Cations : Salt solution + NH4 Cl salt + White ppt. is formed Calcium (Ca+2 ) NH4 OH solution +(NH4 )2 CO3 Barium (Ba+2 ) solution is added Strontium (Sr+2 ) cations are present f) Test for VI Group Cations : Salt solution + NH4 Cl salt + White ppt. is not formed Magnesium (Mg+2 ) NH4 OH solution +(NH4 )2 CO3 + absent Na2 HPO4 solution is added VIII. Confirmation Tests for Barium : a) Action of K2 CrO4 solution : To the salt solution potassium Yellow colour ppt. (BaCrO4 ) is Barium (Ba+2 ) chromate solution (K2 CrO4 ) is formed which is soluble in cation is confirmed. added dil. Acetic acid and soluble in dil HCl b) Action of Ammonium oxalate soution: To the salt solution Ammonium White colour ppt. (BaC2 O4 ) is Barium (Ba+2 ) cation oxalate solution is added. formed. Above ppt. is soluble is confirmed. in hot Acetic acid. IX. Report : The given Cation is : Barium (Ba+2 ) The given Anion is : Bromide (Br– ) The given salt is : Barium Bromide [BaBr2 ]
- 19. eeee 35 Practical Chemistry eeee Janani Publications EXPERIMENT OBSERVATION INFERENCE I. Priliminary Tests : : a) Colour White Cu+2 , Fe+2 , Ni+2 & Mn+2 Cations may be absent b) State Crystalline c) Flame test : Clean the platinum wire by Grassy-green colour flame is Ba+2 May be present dipping in con. HCl and heat observed. it on flame upto no colour appears. Now, prepare the paste of con.HCl + salt in watch glass, then introduce this paste by platinum wire loop into the flame. d) Action of heat : A small quantity of salt is taken Water droplets are formed on the May be Hydrated salt. in a dry test tube and strongly inner wall of test tube. heated. II. Identification of Anion : a) Action of dil.HCl : A small quntity of Salt is taken No reaction Carbonate (CO–2 3 ) & in a dry test tube and dil. HCl Acetate (CH3 COO– ) is added. ions are absent. b) Action of conc.H2 SO4 : A small quantity of salt is Colour less gas (HCl) with Chloride (Cl– ) ion taken in a dry test tube and pungent smell is evolved and may be present Conc.H2 SO4 is added gives dense white fumes (NH4 Cl) with glass rod when dipped in NH4 OH. III. Preparation sodium carbonate extract : To a small amount of the salt, add twice the amount of sodium carbonate, a few ml of distilled water and boil it for five minutes. It is filtered and the filtrate is known as sodium carbonate extract. IV. Confirmation test for Chloride ion : a) Action of MnO2 & Conc. H2 SO4 : To the small quantity of salt is Greenish Yellow colour vapour (Cl2 ) Chloride (Cl– ) ion taken in a test tube pinch of MnO2 with pungent smell are evolved. is confirmed. and Conc.H2 SO4 are added. b) Action of AgNO3 solution : To The salt soluton AgNO3 White curdy ppt. of (AgCl) is formed. Chloride (Cl– ) ion solution is added. Solubility: Above ppt is completely is confirmed. soluble in NH4 OH SALT-10-Barium Chlorides . .
- 20. Practical Chemistry eeee 36 eeee Janani Publications V. Identification of Cation : Preparation of original salt solution : Original salt solution can be prepared by dissolving excess amount of given salt in a suitable solvent. VI. Test for Ammonium (NH+ 4 )ion : Reaction with NaOH : To the small quantity of slat is colour less gas (NH3 ) with pungent Ammonium (NH+ 4 ) taken in a dry test tube a few smell is not evolved. cation is absent. drops of NaOH solution is added and strongly heated VII.Group Separation Table : a) Test for I Group Cations : To the salt solution a few drops of No ppt. is formed. Lead (Pb+2 ) cation is dil HCl solution is added. absent. b) Test for II Group Cations : To the salt solution a few drops of No ppt. is formed. Copper (Cu+2 ) cation dil HCl solution is added and is absent. H2 S gas is passed. c) Test for III Group Cations: To the salt solution NH4 Cl No ppt. is formed. Aluminium (Al+3 ) salt and NH4 OH solution are cation absent added. d) Test for IV Group Cations: Salt solution + NH4 Cl salt + White ppt. ZnS is not formed. Zinc (Zn+2 ) absent NH4 OH solution + H2 S gas passed Flesh colour ppt. MnS is not formed. Manganese (Mn+2 ) absent Black ppt. NiS is not formed. Nickel (Ni+2 ) absent e) Test for V Group Cations : Salt solution + NH4 Cl salt + White ppt. is formed Calcium (Ca+2 ) NH4 OH solution +(NH4 )2 CO3 Barium (Ba+2 ) solution is added Strontium (Sr+2 ) cations are present f) Test for VI Group Cations : Salt solution + NH4 Cl salt + White ppt. is not formed Magnesium (Mg+2 ) NH4 OH solution +(NH4 )2 CO3 + absent Na2 HPO4 solution is added VIII. Confirmation Tests for Barium : a) Action of K2 CrO4 solution : To the salt solution potassium Yellow colour ppt. (BaCrO4 ) is Barium (Ba+2 ) chromate solution (K2 CrO4 ) is formed which is soluble in cation is confirmed. added dil. Acetic acid and soluble in dil HCl b) Action of Ammonium oxalate soution: To the salt solution Ammonium White colour ppt. (BaC2 O4 ) is Barium (Ba+2 ) cation oxalate solution is added. formed. Above ppt. is soluble is confirmed. in hot Acetic acid. IX. Report : The given Cation is : Barium (Ba+2 ) ; The given Anion is : Chloride (Cl– ) The given salt is : Barium Chloride [BaCl2 ]
- 21. eeee 37 Practical Chemistry eeee Janani Publications EXPERIMENT OBSERVATION INFERENCE I. Priliminary Tests : : a) Colour White Cu+2 , Fe+2 , Ni+2 & Mn+2 Cations may be absent b) State Crystalline c) Flame test : Clean the platinum wire by Grassy-green colour flame is Ba+2 May be present dipping in con. HCl and observed. heat it on flame upto no colour appears. Now, prepare the paste of con.HCl + salt in watch glass, then introduce this paste by platinum wire loop into the flame. d) Action of heat : A small quantity of salt is taken Reddish Brown vapours are May be Nitrate (NO3 – ) in a dry test tube and strongly evolved salt is present heated. II. Identification of Anion : a) Action of dil.HCl : A small quntity of Salt is No reaction Carbonate (CO–2 3 ) & taken in a dry test tube and Acetate (CH3 COO– ) dil. HCl is added. ions are absent b) Action of conc.H2 SO4 : A small quantity of salt is a) Before heating no reaction Chloride (Cl– ) & Bromide taken in a dry in a dry test tube b) After heating Reddish (Br– ) ions are absent and conc.H2 SO4 Brown vapours are evolved. Nitrate (NO– 3 ) ion may be present. c) Action of Cu-turnings and conc.H2 SO4 : To the small quantity of salt Reddish Brown vapour of NO2 Nitrate (NO– 3 ) ion present is taken in a test tube 1 or 2 are evolved and the solution pieces of Cu turning and turns pale blue. Conc.H2 SO4 are added and hen heated. III. Preparation sodium carbonate extract : To a small amount of the salt, add twice the amount of sodium carbonate, a few ml of distilled water and boil it for five minutes. It is filtered and the filtrate is known as sodium carbonate extract. IV. Confirmation test for Nitrate ion : a) Brown – Ring test : A small quantity of salt Dark Brown Ring is formed Nitrate (NO– 3 ) ion is solution is taken in a test tube in between the junction of confirmed. and same amount of freshly two layers. (Nitroso ferrous prepared FeSO4 solution is sulphate) (FeSO4 .NO) added, then few drops of conc.H2 SO4 is added by keeping the test tube in an inclined position. SALT-11-Barium Nitrate . .
- 22. Practical Chemistry eeee 38 eeee Janani Publications V. Identification of Cation : Preparation of original salt solution : Original salt solution can be prepared by dissolving excess amount of given salt in a suitable solvent. VI. Test for Ammonium (NH+ 4 )ion : Reaction with NaOH : To the small quantity of slat is colour less gas (NH3 ) with pungent Ammonium (NH+ 4 ) taken in a dry test tube a few smell is not evolved. cation is absent. drops of NaOH solution is added and strongly heated VII.Group Separation Table : a) Test for I Group Cations : To the salt solution a few drops of No ppt. is formed. Lead (Pb+2 ) cation is dil HCl solution is added. absent. b) Test for II Group Cations : To the salt solution a few drops of No ppt. is formed. Copper (Cu+2 ) cation dil HCl solution is added and is absent. H2 S gas is passed. c) Test for III Group Cations: To the salt solution NH4 Cl No ppt. is formed. Aluminium (Al+3 ) salt and NH4 OH solution are cation absent added. d) Test for IV Group Cations: Salt solution + NH4 Cl salt + White ppt. ZnS is not formed. Zinc (Zn+2 ) absent NH4 OH solution + H2 S gas passed Flesh colour ppt. MnS is not formed. Manganese (Mn+2 ) absent Black ppt. NiS is not formed. Nickel (Ni+2 ) absent e) Test for V Group Cations : Salt solution + NH4 Cl salt + White ppt. is formed Calcium (Ca+2 ) NH4 OH solution +(NH4 )2 CO3 Barium (Ba+2 ) solution is added Strontium (Sr+2 ) cations are present f) Test for VI Group Cations : Salt solution + NH4 Cl salt + White ppt. is not formed Magnesium (Mg+2 ) NH4 OH solution +(NH4 )2 CO3 + absent Na2 HPO4 solution is added VIII. Confirmation Tests for Barium : a) Action of K2 CrO4 solution : To the salt solution potassium Yellow colour ppt. (BaCrO4 ) is Barium (Ba+2 ) chromate solution (K2 CrO4 )is formed which is soluble in cation is confirmed. added. dil. Acetic acid and soluble in dil HCl b) Action of Ammonium oxalate soution: To the salt solution Ammonium White colour ppt. (BaC2 O4 ) is Barium (Ba+2 ) cation oxalate solution is added. formed. Above ppt. is soluble is confirmed. in hot Acetic acid. IX. Report : The given Cation is : Barium (Ba+2 ) ; The given Anion is : Nitrate (NO3 – ) The given salt is : Barium Nitrate [Ba(NO3 )2 ]
- 23. eeee 39 Practical Chemistry eeee Janani Publications EXPERIMENT OBSERVATION INFERENCE I. Priliminary Tests : : a) Colour White Cu+2 , Fe+2 , Ni+2 & Mn+2 Cations may be absent b) State Crystalline c) Flame test : Clean the platinum wire Brick red colour flame is Ca+2 May be present by dipping in con.HCl observed and heat it on flame upto no colour appears. Now, prepare the paste of con.HCl + salt in watch glass, then introduce this paste by platinum wire loop into the flame. d) Action of heat : A small quantity of salt is Water droplets are formed May be Hydrated salt. taken in a dry test tube and on the inner walls of test tube. strongly heated. II. Identification of Anion : a) Action of dil HCl : A small quantity of salt is No reaction Carbonate (CO–2 3 ) & taken in a dry test tube and Acetate (CH3 COO– ) ions dil.HCl is added. are absent. b) Action of conc. H2 SO4 : A small quantity of salt is Colour less gas (HCl) with Chloride (Cl– ) ion may be taken in a dry test tube and pungent smell is evolved and present. conc. H2 SO4 is added. gives dense white fumes (NH4 Cl) with glass rod when dipped in NH4 OH. III. Preparation sodium carbonate extract : To a small amount of the salt, add twice the amount of sodium carbonate, a few ml of distilled water and boil it for five minutes. It is filtered and the filtrate is known as sodium carbonate extract. IV. Confirmation test for Chlorides ion : a) Action of MnO2 & Conc. H2 SO4 : To the small quantity of salt is Greenish yellow colour vapour (Cl2 ) Chloride (Cl– ) ion taken in a test tube pinch of MnO2 with pungent smell are evolved. is confirmed. and conc.H2 SO4 are added. b) Action of AgNO3 solution : To the salt solution AgNO3 White curdy ppt. of (AgCl) is Chloride (Cl– ) ion solution is added formed confirmed. Solubility : Above ppt. is completely soluble in NH4 OH SALT-12-Calcium Chloride . .
- 24. Practical Chemistry eeee 40 eeee Janani Publications V. Identification of Cation : Preparation of original salt solution : Original salt solution can be prepared by dissolving excess amount of given salt in a suitable solvent. VI. Test for Ammonium (NH4 + ) ion : Reaction with NaOH : To the small quantity of salt is colourless gas (NH3 ) with Ammonium (NH4 + ) taken in a dry test tube a few drops pungent smell is not evolved. cation is absent of NaOH solution is added and strongly heated. VII. Group Separation Table : a) Test for I Group Cations : To the salt solution a few drops of No ppt. is formed. Lead (Pb+2 ) cation is dil HCl solution is added. absent. b) Test for II Group Cations : To the salt solution a few drops of No ppt. is formed. Copper (Cu+2 ) cation dil HCl solution is added and is absent. H2 S gas is passed. c) Test for III Group Cations: To the salt solution NH4 Cl No ppt. is formed. Aluminium (Al+3 ) salt and NH4 OH solution are cation absent added. d) Test for IV Group Cations: Salt solution + NH4 Cl salt + White ppt. ZnS is not formed. Zinc (Zn+2 ) absent NH4 OH solution + H2 S gas passed Flesh colour ppt. MnS is not formed. Manganese (Mn+2 ) absent Black ppt. Nis is not formed. Nickel (Ni+2 ) absent e) Test for V Group Cations : Salt solution + NH4 Cl salt + White ppt. is formed Calcium (Ca+2 ) NH4 OH solution +(NH4 )2 CO3 Barium (Ba+2 ) solution is added Strontium (Sr+2 ) cations are present f) Test for VI Group Cations : Salt solution + NH4 Cl salt + White ppt. is not formed Magnesium (Mg+2 ) NH4 OH solution +(NH4 )2 CO3 + absent Na2 HPO4 solution is added VIII. Confirmation Tests for Calcium : a) Action of K2 CrO4 solution : To the salt solution potassium Yellow colour ppt. (CaCrO4 ) is Calcium (Ca+2 ) chromate solution (K2 CrO4 ) is not formed. cation is confirmed. added (Yellow colour solution is formed.) b) Action of Ammonium oxalate soution: To the salt solution Ammonium White colour ppt. (CaC2 O4 ) is Calcium (Ca+2 ) cation is oxalate solution is added. formed. Above ppt. is insoluble confirmed. in hot Acetic acid. IX. Report : The given Cation is : Calcium (Ca+2 ) ; The given Anion is : Chloride (Cl– ) The given salt is : Calcium Chloride [CaCl2 ]
- 25. eeee 41 Practical Chemistry eeee Janani Publications SALT-13-Calcium Nitrate . . EXPERIMENT OBSERVATION INFERENCE I. Priliminary Tests : : a) Colour White Cu+2 , Fe+2 , Ni+2 & Mn+2 Cations may be absent b) State Crystalline c) Flame test : Clean the platinum wire by Brick red colour flame is Ca+2 May be present ipping in con.HCl and heat observed. it on flame upto no colour appears. Now, prepare the paste of conc.HCl + salt in watch glass, then introduce this paste by platinum wire loop into the flame. d) Action of heat : A small quantity of salt is Reddish Brown vapours are May be Nitrate (NO– 3 ) taken in a dry test tube and evolved. salt is present. strongly heated. II. Identification of Anion : a) Action of dil. HCl : A small quantity of salt is No reaction Carbonate (CO–2 3 ) & taken in a dry test tube and Acetate (CH3 COO– ) ions dil.HCl is added. are absent. b) Action of conc.H2 SO4 : A small quantity of salt is a) Before heating no reaction Chloride (Cl– ) & Bromide taken in a dry test tube b) After heating Reddish Brown (Br– ) ions are absent and conc.H2 SO4 is added vapours are evolved. Nitrate (NO– 3 ) ion may be present. c) Action of Cu turnings and conc.H2 SO4 : To the small quantity of salt Reddish Brown vapours of NO2 are Nitrate (NO– 3 ) ion is is taken in a test tube 1 or 2 evolved and the solution turns pale present pieces of Cu turnings and blue Conc. H2 SO4 are added and then heated. III. Preparation sodium carbonate extract : To a small amount of the salt, add twice the amount of sodium carbonate, a few ml of distilled water and boil it for five minutes. It is filtered and the filtrate is known as sodium carbonate extract. IV. Confirmation test for Nitrate ion : a) Brown – Ring test : A small quantity of salt Dark Brown Ring is formed Nitrate (NO– 3 ) ion is solution is taken in a test tube in between the junction of confirmed. and same amount of freshly two layers. (Nitroso ferrous prepared FeSO4 solution is sulphate) (FeSO4 .NO) added, then few drops of conc.H2 SO4 is added by keeping the test tube in an inclined position.
- 26. Practical Chemistry eeee 42 eeee Janani Publications V. Identification of Cation : Preparation of original salt solution : Original salt solution can be prepared by dissolving excess amount of given salt in a suitable solvent. VI. Test for Ammonium (NH+ 4 )ion : Reaction with NaOH : To the small quantity of slat is colour less gas (NH3 ) with Ammonium (NH+ 4 ) taken in a dry test tube a few pungent smell is not evolved. cation is absent. drops of NaOH solution is added and strongly heated VII. Group Separation Table : a) Test for I Group Cations : To the salt solution a few drops of No ppt. is formed. Lead (Pb+2 ) cation is dil HCl solution is added. absent. b) Test for II Group Cations : To the salt solution a few drops of No ppt. is formed. Copper (Cu+2 ) cation dil HCl solution is added and is absent. H2 S gas is passed. c) Test for III Group Cations: To the salt solution NH4 Cl No ppt. is formed. Aluminium (Al+3 ) salt and NH4 OH solution are cation absent added. d) Test for IV Group Cations: Salt solution + NH4 Cl salt + White ppt. ZnS is not formed. Zinc (Zn+2 ) absent NH4 OH solution + H2 S gas passed Flesh colour ppt. MnS is not formed. Manganese (Mn+2 ) absent Black ppt. NiS is not formed. Nickel (Ni+2 ) absent e) Test for V Group Cations : Salt solution + NH4 Cl salt + White ppt. is formed Calcium (Ca+2 ) NH4 OH solution +(NH4 )2 CO3 Barium (Ba+2 ) solution is added Strontium (Sr+2 ) cations are present f) Test for VI Group Cations : Salt solution + NH4 Cl salt + White ppt. is not formed Magnesium (Mg+2 ) NH4 OH solution +(NH4 )2 CO3 + cation is absent Na2 HPO4 solution is added VIII. Confirmation Tests for Calcium : a) Action of K2 CrO4 solution : To the salt solution potassium Yellow colour ppt. (CaCrO4 ) is Calcium (Ca+2 ) cation chromate solution (K2 CrO4 ) not formed. is confirmed. is added (Yellow colour solution is formed) b) Action of Ammonium oxalate solution : To the salt solution Ammonium White colour ppt. (CaC2 O4 ) is Calcium (Ca+2 ) cation oxalate solution is added formed. Above ppt. is insoluble confirmed. in hot Acetic acid. IX. Report : The given Cation is : Calcium (Ca+2 ) ; The given Anion is : Nitrate (NO– 3 ) The given salt is : Calcium Nitrate [Ca (NO3 )2 ]
- 27. eeee 43 Practical Chemistry eeee Janani Publications EXPERIMENT OBSERVATION INFERENCE I. Priliminary Tests : : a) Colour Blue May be Cu+2 cation is present b) State Crystalline c) Flame test : Clean the platinum wire Bright bluish-green colour Cu+2 May be present by dipping in con. HCl flame is observed. and heat it on flame upto no colour appears. Now, prepare the paste of con.HCl + salt in watch glass, then introduce this paste by platinum wire loop into the flame. d) Action of heat : small quantity of salt is Reddish Brown vapours are May be Nitrate (NO– 3 ) taken in a dry test tube evolved. salt is present. and strongly heated. II. Identification of Anion : a) Action of dil HCl : A small quantity of salt is No reaction Carbonate (CO–2 3 ) & taken in a dry test tube and Acetate (CH3 COO– ) ions dil.HCl is added. are absent. b) Action of Conc. H2 SO4 : To the small quantity of salt a) Before heating no Reaction. Chloride (Cl– ) & Bromide is taken in a test tube and (Br– ) ions are absent conc.H2 SO4 is added. b) After heating Reddish Brown Nitrate (NO– 3 ) ion vapours are evolved may be present c) Action of Cu-turnings and Conc.H2 SO4 : To the small quantity of salt Reddish Brown vapours of NO2 are Nitrate (NO– 3 ) ion is is taken in a test tube 1 or 2 evolved and the solution turns pale present pieces of Cu-turnings and blue. conc.H2 SO4 are added and then heated. III. Preparation sodium carbonate extract : To a small amount of the salt, add twice the amount of sodium carbonate, a few ml of distilled water and boil it for five minutes. It is filtered and the filtrate is known as sodium carbonate extract. IV. Confirmation test for Nitrate ion : a) Brown – Ring test : A small quantity of salt Dark Brown Ring is formed Nitrate (NO– 3 ) ion is solution is taken in a test tube in between the junction of confirmed. and same amount of freshly two layers. (Nitroso ferrous prepared FeSO4 solution is added, sulphate) (FeSO4 .NO) then few drops of conc.H2 SO4 is added by keeping the test tube in an inclined position. SALT-14-Copper Nitrate . .
- 28. Practical Chemistry eeee 44 eeee Janani Publications V. Identification of Cation : Preparation of original salt solution : Original salt solution can be prepared by dissolving excess amount of given salt in a suitable solvent. VI. Test for Ammonium (NH+ 4 )ion : Reaction with NaOH : To the small quantity of slat is colour less gas (NH3 ) with Ammonium (NH+ 4 ) taken in a dry test tube a few pungent smell is not evolved. cation is absent. drops of NaOH solution is added and strongly heated VII. Group Separation Table : a) Test for I Group Cations : To the salt solution a few drops of No ppt. is formed. Lead (Pb+2 ) cation is dil HCl solution is added. absent. b) Test for II Group Cations : To the salt solution a few drops of Blue ppt. is formed. Copper (Cu+2 ) cation dil HCl solution is added and is present. H2 S gas is passed. c) Test for III Group Cations: To the salt solution NH4 Cl No ppt. is formed. Aluminium (Al+3 ) salt and NH4 OH solution are cation is absent. added. d) Test for IV Group Cations: Salt solution + NH4 Cl salt + White ppt. ZnS is not formed. Zinc (Zn+2 ) absent NH4 OH solution + H2 S gas passed Flesh colour ppt. MnS is not formed. Manganese (Mn+2 ) absent Black ppt. NiS is not formed. Nickel (Ni+2 ) absent e) Test for V Group Cations: Salt solution + NH4 Cl salt + White ppt. CaCO3 is not formed. Calcium (Ca+2 )absent NH4 OH solution +(NH4 )2 CO3 White ppt. BaCO3 is not formed. Barium (Ba+2 ) absent solution is added White ppt. SrCO3 is not formed. Strontium (Sr+2 ) absent f) Test for VI Group Cations : Salt solution + NH4 Cl salt + White ppt. is not formed Magnesium (Mg+2 ) NH4 OH solution +(NH4 )2 CO3 + absent Na2 HPO4 solution is added VIII. Confirmation Tests for Copper : a) Action of NaOH solution : To the salt solution NaOH Blue colour ppt. is formed. Copper (Cu+2 ) cation is solution is added [Cu(OH)2 ] confirmed. b) Action of K4 [Fe(CN)6 ] solution : To the salt solution Potassium Chocolate colour ppt. is formed. Copper (Cu+2 ) cation ferrocyanide (K4 [Fe(CN)6 ]) Cu2 [Fe(CN)6 ] is confirmed. solution is added. Which is soluble in dil.HCl. IX. Report : The given Cation is : Copper (Cu+2 ) The given Anion is : Nitrate (NO– 3 ) The given salt is : Copper Nitrate [Cu (NO3 )2 ]
- 29. eeee 45 Practical Chemistry eeee Janani Publications EXPERIMENT OBSERVATION INFERENCE I. Priliminary Tests : : a) Colour Blue May be Cu+2 cation is present b) State Crystalline c) Flame test : Clean the platinum wire Bright bluish-green colour Cu+2 may be present by dipping in con. HCl flame is observed. and heat it on flame upto no colour appears. Now, prepare the paste of con.HCl + salt in watch glass, then introduce this paste by platinum wire loop into the flame. d) Action of heat : A small quantity of salt is Water droplets are formed on May be Hydrated salt. taken in a dry test tube and the inner walls of test tube. strongly heated. II. Identification of Anion : a) Action of dil HCl : A small quantity of salt is No reaction Carbonate (CO–2 3 ) & aken in a dry test tube and Acetate (CH3 COO– ) ions dil.HCl is added. are absent. b) Action of Conc. H2 SO4 : A small quantity of salt is a) Before heating no Reaction. Chloride (Cl– )& Brominde taken in a dry test tube and (Br– ) ions are bsent. Conc.H2 SO4 is added. b) After heating no reaction Nitrate (NO– 3 ) ion is absent c) Action of Cu-turning and Conc.H2 SO4 : To the small quantity of salt No reaction Nitrate (NO– 3 ) ion is absent is taken in a test tube 1 or 2 pieces of Cu turning and Conc.H2 SO4 are added and then heated. d) Action of BaCl2 solution : To the salt solution BaCl2 White ppt. of (BaSO4 ) is formed. Sulphate (SO–2 4 ) ion is solution is added. present. III. Preparation sodium carbonate extract : To a small amount of the salt, add twice the amount of sodium carbonate, a few ml of distilled water and boil it for five minutes. It is filtered and the filtrate is known as sodium carbonate extract. SALT-15 - Copper Sulphate . .
- 30. Practical Chemistry eeee 46 eeee Janani Publications IV. Confirmation test for Sulphate ion : Action of BaCl2 solution : To the salt solution BaCl2 White ppt. of (BaSO4 ) is formed. Sulphate (SO–2 4 ) ion is solution is added. Solubility: Above ppt. is insoluble confirmed. in conc.HCl. V. Identification of Cation : Preparation of original salt solution : Original salt solution can be prepared by dissolving excess amount of given salt in a suitable solvent. VI. Test for Ammonium (NH+ 4 )ion : Reaction with NaOH : To the small quantity of slat is colour less gas (NH3 ) with Ammonium (NH+ 4 ) taken in a dry test tube a few pungent smell is not evolved. cation is absent. drops of NaOH solution is added and strongly heated VII. Group Separation Table : a) Test for I Group Cations : To the salt solution a few drops of No ppt. is formed. Lead (Pb+2 ) cation is dil HCl solution is added. absent. b) Test for II Group Cations : To the salt solution a few drops of Blue ppt. is formed. Copper (Cu+2 ) cation dil HCl solution is added and is present. H2 S gas is passed. c) Test for III Group Cations: To the salt solution NH4 Cl salt No ppt. is formed. Aluminium (Al+3 ) and NH4 OH solution are added. cation is absent. d) Test for IV Group Cations: Salt solution + NH4 Cl salt + White ppt. ZnS is not formed. Zinc (Zn+2 ) absent NH4 OH solution + H2 S gas passed Flesh colour ppt. MnS is not formed. Manganese (Mn+2 ) absent Black ppt. Nis is not formed. Nickel (Ni+2 ) absent e) Test for V Group Cations: Salt solution + NH4 Cl salt + White ppt. CaCO3 is not formed. Calcium (Ca+2 ) absent NH4 OH solution +(NH4 )2 CO3 White ppt. BaCO3 is not formed. Barium (Ba+2 ) absent solution is added White ppt. SrCO3 is not formed. Strontium (Sr+2 ) absent f) Test for VI Group Cations : Salt solution + NH4 Cl salt + White ppt. is not formed Magnesium (Mg+2 ) NH4 OH solution +(NH4 )2 CO3 + absent Na2 HPO4 solution is added VIII. Confirmation Tests for Copper : a) Action of NaOH solution : To the salt solution NaOH Blue colour ppt. is formed. Copper (Cu+2 ) cation is solution is added [Cu(OH)2 ] confirmed. b) Action of K4 [Fe(CN)6 ] solution : To the salt solution Potassium Chocolate colour ppt. is formed. Copper (Cu+2 ) cation ferrocyanide (K4 [Fe(CN)6 ]) Cu2 [Fe(CN)6 ] is confirmed. solution is added. Which is soluble in dil.HCl. IX. Report : The given Cation is : Copper (Cu+2 ) ; The given Anion is : Sulphate (SO–2 4 ) The given salt is : Copper Sulphate [CuSO4 ]
- 31. eeee 47 Practical Chemistry eeee Janani Publications SALT-16 - Ferrous Sulphate . . EXPERIMENT OBSERVATION INFERENCE I. Priliminary Tests : : a) Colour : Pale Green May be Fe+2 cation is present b) State : Crystal line c)Flame test : Clean the platinum wire by Colour flame is not observed Ca+2 ,Sr+2 ,Ba+2 ,Cu+2 ,Zn+2 dipping in con. HCl and heat (or) Mn+2 , pb+2 are absent. it on flame upto no colour appears. Now, prepare the paste of con.HCl + salt in watch glass, then introduce this paste by platinum wire loop into the flame. d) Action of heat : A small quantity of salt is taken Water droplets are formed on May be Hydrated salt. in a dry test tube and strongly the inner walls of test tube. heated. II. Identification of Anion : a) Action of dil HCl : A small quantity of salt is No reaction Carbonate (CO–2 3 ) & taken in a dry test tube and Acetate (CH3 COO– ) ions dil.HCl is added. are absent. b) Action of Conc. H2 SO4 : A small quantity of salt is a) Before heating no Reaction. Chloride (Cl– ) & taken in a dry test tube and Brominde (Br– ) Conc.H2 SO4 is added. b) After heating no reaction ions are bsent. Nitrate (NO– 3 ) ion is absent c) Action of Cu-turning and Conc.H2 SO4 : To the small quantity of No reaction Nitrate (NO– 3 ) ion is absent salt is taken in a test tube 1 or 2 pieces of Cu turning and conc.H2 SO4 are added and then heated. d) Action of BaCl2 solution : To the salt solution BaCl2 White ppt. of (BaSO4 ) is formed. Sulphate (SO–2 4 ) ion is solution is added. present. III. Preparation sodium carbonate extract : To a small amount of the salt, add twice the amount of sodium carbonate, a few ml of distilled water and boil it for five minutes. It is filtered and the filtrate is known as sodium carbonate extract. IV. Confirmation test for Sulphate ion : Action of BaCl2 solution : To the salt solution BaCl2 White ppt. of (BaSO4 ) is formed. Sulphate (SO–2 4 ) ion is solution is added. Solubility: Above ppt. is insoluble confirmed. in conc.HCl.
- 32. Practical Chemistry eeee 48 eeee Janani Publications V. Identification of Cation : Preparation of original salt solution : Original salt solution can be prepared by dissolving excess amount of given salt in a suitable solvent. VI. Test for Ammonium (NH+ 4 )ion : Reaction with NaOH : To the small quantity of slat is colour less gas (NH3 ) with Ammonium (NH+ 4 ) taken in a dry test tube a few pungent smell is not evolved. cation is absent. drops of NaOH solution is added and strongly heated VII. Group Separation Table : a) Test for I Group Cations : To the salt solution a few drops of No ppt. is formed. Lead (Pb+2 ) cation is dil HCl solution is added. absent. b) Test for II Group Cations : To the salt solution a few drops of No ppt. is formed. Copper (Cu+2 ) cation dil HCl solution is added and is absent. H2 S gas is passed. c) Test for III Group Cations: To the salt solution NH4 Cl Dirty green ppt. is formed. Ferrous (Fe+2 ) salt and NH4 OH solution are cation is present added. d) Test for IV Group Cations: Salt solution + NH4 Cl salt + White ppt. ZnS is not formed. Zinc (Zn+2 ) absent NH4 OH solution + H2 S gas passed Flesh colour ppt. MnS is not formed. Manganese (Mn+2 ) absent Black ppt. Nis is not formed. Nickel (Ni+2 ) absent e) Test for V Group Cations: Salt solution + NH4 Cl salt + White ppt. CaCO3 is not formed. Calcium (Ca+2 )absent NH4 OH solution +(NH4 )2 CO3 White ppt. BaCO3 is not formed. Barium (Ba+2 ) absent solution is added White ppt. SrCO3 is not formed. Strontium (Sr+2 ) absent f) Test for VI Group Cations : Salt solution + NH4 Cl salt + White ppt. is not formed Magnesium (Mg+2 ) NH4 OH solution +(NH4 )2 CO3 + absent Na2 HPO4 solution is added VIII. Confirmation Tests for Ferrous : a) Action of NH4 OH solution : To the salt solution NH4 OH Dirty Green ppt. [Fe(OH)2 ] is Ferrous (Fe+2 ) cation is solution is added. formed. confirmed. b)Action of K4 [Fe(CN)6 ] solution : To the salt solution Potassium Deep Blue ppt. is formed. Ferrous (Fe+2 ) cation ferrocyanide (K4 [Fe(CN)6 ]) is confirmed. solution is added. IX. Report : The given Cation is : Ferrous (Fe+2 ) The given Anion is : Sulphate (SO–2 4 ) The given salt is : Ferrous sulphate [FeSO4 ]
- 33. eeee 49 Practical Chemistry eeee Janani Publications SALT-17 - Lead Nitrate . . EXPERIMENT OBSERVATION INFERENCE I. Priliminary Tests : : a) Colour White Cu+2 , Fe+2 , Ni+2 & Mn+2 Cations may be absent b) State Crystalline c) Flame test : Clean the platinum wire Dull blusish-white colour flame Pb+2 maybe present by dipping in con. HCl is observed and heat it on flame upto no colour appears. Now, prepare the paste of con.HCl + salt in watch glass, then introduce this paste by platinum wire loop into the flame. d) Action of heat : A small quantity of salt is Cracking sound is observed. May be Lead Salt taken in a dry test tube and is present. strongly heated. II. Identification of Anion : a) Action of dil HCl : A small quantity of salt is No reaction. Carbonate (CO–2 3 ) & taken in a dry test tube and Acetate (CH3 COO– ) dil.HCl is added. ions are absent. b) Action of Conc.H2 SO4 : A small quantity of salt is a) Before heating no reaction. Chloride (Cl– ) & Bromide taken in a dry test tube and (Br– )ions are absent. conc.H2 SO4 is added. b) After heating reddish Brown Nitrate(NO3 – ) ion vapours are evolved. may be present Action of Cu-turning and Conc.H2 SO4 : To the small quantity of Reddish Brown vapours of Nitrate (NO– 3 ) ion is salt is taken in a test tube NO2 are evolved and the solution present. 1 or 2 pieces of Cu turnings turns pale blue and conc.H2 SO4 are added and then heated. III. Preparation sodium carbonate extract : To a small amount of the salt, add twice the amount of sodium carbonate, a few ml of distilled water and boil it for five minutes. It is filtered and the filtrate is known as sodium carbonate extract.
- 34. Practical Chemistry eeee 50 eeee Janani Publications IV. Confirmation test for Nitrate ion : a) Brown – Ring test : A small quantity of salt solution Dark Brown Ring is formed in Nitrate (NO– 3 ) ion is is taken in a test tube and same between the junction of two layers. confirmed. amount of freshly prepared FeSO4 (Nitroso ferrous sulphate) solution is added, then few drops (FeSO4 .NO) of conc. H2 SO4 is added by keeping the test tube in an inclined position. V. Identification of Cation : Preparation of original salt solution : Original salt solution can be prepared by dissolving excess amount of given salt in a suitable solvent. VI. Test for Ammonium (NH+ 4 )ion : Reaction with NaOH : To the small quantity of slat is colour less gas (NH3 ) with Ammonium (NH+ 4 ) taken in a dry test tube a few pungent smell is not evolved. cation is absent. drops of NaOH solution is added and strongly heated VII. Group Separation Table : a) Test for I Group Cations : To the salt solution a few drops of White ppt. is formed. Lead (Pb+2 ) cation is dil HCl solution is added. present. b) Test for II Group Cations : To the salt solution a few drops of No ppt. is formed. Copper (Cu+2 ) cationdil HCl solution is added and is absent. H2 S gas is passed. c) Test for III Group Cations: To the salt solution NH4 Cl No ppt. is formed. Aluminium (Al+3 ) salt and NH4 OH solution are cation is absent. added. d) Test for IV Group Cations: Salt solution + NH4 Cl salt + White ppt. ZnS is not formed. Zinc (Zn+2 ) absent NH4 OH solution + H2 S gas passed Flesh colour ppt. MnS is not formed. Manganese (Mn+2 ) absent Black ppt. Nis is not formed. Nickel (Ni+2 ) absent e) Test for V Group Cations: Salt solution + NH4 Cl salt + White ppt. CaCO3 is not formed. Calcium (Ca+2 ) absent NH4 OH solution +(NH4 )2 CO3 White ppt. BaCO3 is not formed. Barium (Ba+2 ) absent solution is added White ppt. SrCO3 is not formed. Strontium (Sr+2 ) absent f) Test for VI Group Cations : Salt solution + NH4 Cl salt + White ppt. is not formed Magnesium (Mg+2 ) NH4 OH solution +(NH4 )2 CO3 + absent Na2 HPO4 solution is added VIII. Confirmation Tests for Lead : a) Action of K2 CrO4 solution : To the salt solution Potassium Yellow colour ppt. (PbCrO4 ) is Lead (Pb+2 ) cation chromate solution (K2 CrO4 ) formed. Which dissolves in is confirmed. is added. NaOH solution b)Action KI solution: To the salt solution Potassium A Yellow colour ppt. is formed Lead (Pb+2 ) cation is iodided (KI) solution is added. which is disolved in hot water confirmed. and cooled. Golden yellow spangles are formed. IX. Report : The given Cation is : Lead (Pb+2 ) ; The given Anion is : Nitrate (NO– 3 ) The given salt is : Lead Nitrate [Pb(NO3 )2 ]
- 35. eeee 51 Practical Chemistry eeee Janani Publications EXPERIMENT OBSERVATION INFERENCE I. Priliminary Tests : : a) Colour White Cu+2 , Fe+2 , Ni+2 & Mn+2 Cations may be absent b) State Crystalline c) Flame test : Clean the platinum wire Colour flame is not observed Ca+2 , Sr+2 , Ba+2 , Cu+2 , Zn+2 by dipping in con. HCl (or) Mn+2 , Pb+2 are absent. and heat it on flame upto no colour appears. Now, prepare the paste of con.HCl + salt in watch glass, then introduce this paste by platinum wire loop into the flame. d)Action of heat : A small quantity of salt is Water droplete are formed on the May be Hydrated salt taken in a dry test tube and inner walls of test tube. strongly heated. II. Identification of Anion : a) Action of dil HCl : A small quantity of salt is No reaction. Carbonate (CO–2 3 ) & taken in a dry test tube and Acetate (CH3 COO– ) dil. HCl is added. ions are absent. b) Action of Conc.H2 SO4 : A small quantity of salt is Colour less gas (HCl). with Chloride (Cl– ) ion taken in a dry test tube and pungent smell is evolved and may be present. conc.H2 SO4 is added. gives dense white fumes (NH4 Cl) with glass rod when dipped in NH4 OH III. Preparation sodium carbonate extract : To a small amount of the salt, add twice the amount of sodium carbonate, a few ml of distilled water and boil it for five minutes. It is filtered and the filtrate is known as sodium carbonate extract. IV. Confirmation test for Chloride ion : 1. Action of MnO2 & conc. H2 SO4 : To the small quantity of salt is Greenish yellow colour vapour (Cl2 ) Chloride (Cl– ) ion is taken in a test tube pinch of with pungent smell are evolved. confirmed. MnO2 and conc.H2 SO4 are added. 2. Action of AgNO3 solution : To the salt solution AgNO3 White curd ppt. of (AgCl) is formed. Chloride (Cl– ) ion is solution is added. Solubility : Above ppt. is completely confirmed. soluble in NH4 OH SALT-18 - Magnesium Chloride . .
- 36. Practical Chemistry eeee 52 eeee Janani Publications V. Identification of Cation : Preparation of original salt solution : Original salt solution can be prepared by dissolving excess amount of given salt in a suitable solvent. VI. Test for Ammonium (NH+ 4 )ion : Reaction with NaOH : To the small quantity of slat is colour less gas (NH3 ) with Ammonium (NH+ 4 ) taken in a dry test tube a few pungent smell is not evolved. cation is absent. drops of NaOH solution is added and strongly heated VII. Group Separation Table : a) Test for I Group Cations : To the salt solution a few drops of No ppt. is formed. Lead (Pb+2 ) cation is dil HCl solution is added. absent. b) Test for II Group Cations : To the salt solution a few drops of No ppt. is formed. Copper (Cu+2 ) cation dil HCl solution is added and is absent. H2 S gas is passed. c) Test for III Group Cations: To the salt solution NH4 Cl No ppt. is formed. Aluminium (Al+3 ) salt and NH4 OH solution are cation is absent added. d) Test for IV Group Cations: Salt solution + NH4 Cl salt + White ppt. ZnS is not formed. Zinc (Zn+2 ) absent NH4 OH solution + H2 S gas passed Flesh colour ppt. MnS is not formed. Manganese (Mn+2 ) absent Black ppt. Nis is not formed. Nickel (Ni+2 ) absent e) Test for V Group Cations: Salt solution + NH4 Cl salt + White ppt. CaCO3 is not formed. Calcium (Ca+2 )absent NH4 OH solution +(NH4 )2 CO3 White ppt. BaCO3 is not formed. Barium (Ba+2 ) absent solution is added White ppt. SrCO3 is not formed. Strontium (Sr+2 ) absent f) Test for VI Group Cations : Salt solution + NH4 Cl salt + White ppt. is formed Magnesium (Mg+2 ) NH4 OH solution +(NH4 )2 CO3 + cation is present Na2 HPO4 solution is added VIII. Confirmation Tests for Magnesium : a) Action of NaOH solution: To the salt solution NaOH white ppt. is formed. Magnesium (Mg+2 ) cation is solution is added Which is insoluble in excess confirmed. of NaOH. b) To the salt solution Na2 CO3 White ppt. is formed. Magnesium (Mg+2 ) cation is solution is added confirmed. IX. Report : The given Cation is : Magnesium (Mg+2 ) The given Anion is : Chloride (Cl– ) The given salt is : Magnesium Chloride [MgCl2 ]
- 37. eeee 53 Practical Chemistry eeee Janani Publications SALT-19 - Magnesium Sulphate . . EXPERIMENT OBSERVATION INFERENCE I. Priliminary Tests : : a) Colour White Cu+2 , Fe+2 , Ni+2 & Mn+2 Cations may be absent b) State Crystalline c) Flame test : Clean the platinum wire Colour flame is not observed Ca+2 , Sr+2 , Ba+2 , Cu+2 , Zn+2 by dipping in con. HCl (or) Mn+2 , Pb+2 are absent. and heat it on flame upto no colour appears. Now, prepare the paste of con.HCl + salt in watch glass, then introduce this paste by platinum wire loop into the flame. d)Action of heat : A small quantity of salt is Water droplets are formed on the May be Hydrated salt taken in a dry test tube and inner walls of test tube. strongly heated. II. Identification of Anion : a) Action of dil HCl : A small quantity of salt is No reaction. Carbonate (CO–2 3 ) & taken in a dry test tube and Acetate (CH3 COO– ) dil. HCl is added. ions are absent. b) Action of Conc.H2 SO4 : A small quantity of salt is a) Before heating no reaction. Chloride (Cl) & bromide taken in a dry test tube and b) After heating no reaction (Br– ) Nitrate (NO– 3 ) ion is conc.H2 SO4 is added. absent. c) Action of Cu-turnings and Conc. H2 SO4 : To the small quantity of No reaction Nitrate (NO– 3 ) ion is absent salt is taken in a test tube 1 or 2 pieces of Cu turnings and conc.H2 SO4 are added and then heated. d) Action of BaCl2 solution : To the salt solution BaCl2 White ppt. (BaSO4 ) is formed. Sulphate (SO–2 4 ) ion is solution is added. present. III. Preparation sodium carbonate extract : To a small amount of the salt, add twice the amount of sodium carbonate, a few ml of distilled water and boil it for five minutes. It is filtered and the filtrate is known as sodium carbonate extract.
- 38. Practical Chemistry eeee 54 eeee Janani Publications IV. Confirmation test for Sulphate ion : Action of BaCl2 solution : To the salt solution BaCl2 White ppt. of (BaSO4 ) is formed. Sulphate (SO–2 4 ) ion is solution is added. Solubility: Above ppt. is insoluble confirmed. in conc.HCl. V. Identification of Cation : Preparation of original salt solution : Original salt solution can be prepared by dissolving excess amount of given salt in a suitable solvent. VI. Test for Ammonium (NH+ 4 )ion : Reaction with NaOH : To the small colour less gas (NH3 ) with Ammonium (NH+ 4 ) quantity of salt is taken in a dry test pungent smell is not evolved. cation is absent. tube few drops of NaOH solution is added and heated strongly. VII. Group Separation Table : a) Test for I Group Cations : To the salt solution a few drops of No ppt. is formed. Lead (Pb+2 ) cation is dil HCl solution is added. absent. b) Test for II Group Cations : To the salt solution a few drops of No ppt. is formed. Copper (Cu+2 ) cation dil HCl solution is added and is absent. H2 S gas is passed. c) Test for III Group Cations: To the salt solution NH4 Cl No ppt. is formed. Aluminium (Al+3 ) salt and NH4 OH solution are cation is absent added. d) Test for IV Group Cations: Salt solution + NH4 Cl salt + White ppt. ZnS is not formed. Zinc (Zn+2 ) absent NH4 OH solution + H2 S gas passed Flesh colour ppt. MnS is not formed. Manganese (Mn+2 ) absent Black ppt. Nis is not formed. Nickel (Ni+2 ) absent e) Test for V Group Cations: Salt solution + NH4 Cl salt + White ppt. CaCO3 is not formed. Calcium (Ca+2 )absent NH4 OH solution +(NH4 )2 CO3 White ppt. BaCO3 is not formed. Barium (Ba+2 ) absent solution is added White ppt. SrCO3 is not formed. Strontium (Sr+2 ) absent f) Test for VI Group Cations : Salt solution + NH4 Cl salt + White ppt. is formed Magnesium (Mg+2 ) NH4 OH solution +(NH4 )2 CO3 + cation is present Na2 HPO4 solution is added VIII. Confirmation Tests for Magnesium : a) Action of NaOH solution : To the salt solution NaOH white ppt. is formed. Magnesium (Mg+2 ) solution is added Which is insoluble in excess cation is confirmed. of NaOH. b) To the salt solution Na2 CO3 White ppt. is formed. Magnesium (Mg+2 ) solution is added cation is confirmed. IX. Report : The given Cation is : Magnesium (Mg+2 ) The given Anion is : Sulphate (SO–2 4 ) The given salt is : Magnesium Sulphate [MgSO4 ]
- 39. eeee 55 Practical Chemistry eeee Janani Publications EXPERIMENT OBSERVATION INFERENCE I. Priliminary Tests : : a) Colour Pale pink Mn+2 cation may be present b) State Crystalline c) Flame test : Clean the platinum wire Colour flame is not observed Zn+2 (or) Mn+2 , may be by dipping in con. HCl present and heat it on flame upto no colour appears. Now, prepare the paste of con.HCl + salt in watch glass, then introduce this paste by platinum wire loop into the flame. d)Action of heat : A small quantity of salt is Water droplets are formed on the May be Hydrated salt taken in a dry test tube and inner walls of test tube. strongly heated. II. Identification of Anion : a) Action of dil HCl : A small quantity of salt is No reaction. Carbonate (CO–2 3 ) & taken in a dry test tube and Acetate (CH3 COO– ) dil. HCl is added. ions are absent. ions are absent. b) Action of conc.H2 SO4 : A small quantity of salt is Colour less gas (HCl) with Chloride (Cl– ) ion taken in a dry test tube pungent smell is evolved may be present. and conc.H2 SO4 is added. and gives dense white fumes (NH4 Cl) with glass rod when dipped in NH4 OH III. Preparation sodium carbonate extract : To a small amount of the salt, add twice the amount of sodium carbonate, a few ml of distilled water and boil it for five minutes. It is filtered and the filtrate is known as sodium carbonate extract. IV. Confirmation test for Chloride ion : a)Action of MnO2 & Conc.H2 SO4 : To the small quantity of salt is Greenish yellow colour Chloride (Cl– ) ion is taken in a test tube pinch of MnO2 vapours (Cl2 ) with pungent confirmed. and conc.H2 SO4 are added. smell are evolved. b)Action of AgNO3 Solution : To the salt solution AgNO3 White curd ppt. of (AgCl) is Chloride (Cl– ) ion is solution is added. formed. confirmed. Solubility : Above ppt. is completely soluble in NH4 OH SALT-20 - Manganese Chloride . .
- 40. Practical Chemistry eeee 56 eeee Janani Publications V. Identification of Cation : Preparation of original salt solution : Original salt solution can be prepared by dissolving excess amount of given salt in a suitable solvent. VI. Test for Ammonium (NH+ 4 )ion : Reaction with NaOH : To the small colour less gas (NH3 ) with Ammonium (NH+ 4 ) quantity of salt is taken in a dry test pungent smell is not evolved. cation is absent. tube few drops of NaOH solution is added and heated strongly. VII.Group Separation Table : a) Test for I Group Cations : To the salt solution a few drops of No ppt. is formed. Lead (Pb+2 ) cation is dil HCl solution is added. absent. b) Test for II Group Cations : To the salt solution a few drops of No ppt. is formed. Copper (Cu+2 ) cation dil HCl solution is added and is absent. H2 S gas is passed. c) Test for III Group Cations: To the salt solution NH4 Cl No ppt. is formed. Aluminium (Al+3 ) salt and NH4 OH solution are cation is absent. added. d) Test for IV Group Cations: Salt solution + NH4 Cl salt + NH4 OH solution + H2 S gas passed Flesh colour ppt. MnS is formed. Manganese (Mn+2 ) cation is present. e) Test for V Group Cations: Salt solution + NH4 Cl salt + White ppt. CaCO3 is not formed. Calcium (Ca+2 )absent NH4 OH solution +(NH4 )2 CO3 White ppt. BaCO3 is not formed. Barium (Ba+2 ) absent solution is added White ppt. SrCO3 is not formed. Strontium (Sr+2 ) absent f) Test for VI Group Cations : Salt solution + NH4 Cl salt + White ppt. is not formed Magnesium (Mg+2 ) NH4 OH solution +(NH4 )2 CO3 + absent Na2 HPO4 solution is added VIII. Confirmation Tests for Magnesium : a)Action of NaOH solution : To the salt solution Sodium White ppt. Mn (OH)2 is formed. Manganes (Mn+2 ) cation is Hydroxide solution (NaOH) Which turns to Brown colour. confirmed. is added. It is insoluble in excess of NaOH solution. b) Action of NH4 OH solution : To the salt solution NH4 OH White ppt. Mn (OH)2 is formed. Manganese (Mn+2 ) cation solution is added Which is soluble in excess of is confirmed. NH4 OH solution. IX. Report : The given Cation is : Manganese (Mn+2 ) The given Anion is : Chloride (Cl– ) The given salt is : Manganese Chloride [MnCl2 ]
- 41. eeee 57 Practical Chemistry eeee Janani Publications SALT-21 - Manganese Sulphate . . EXPERIMENT OBSERVATION INFERENCE I. Priliminary Tests : : a) Colour : Pale pink Mn+2 cation may be present b) State : Crystalline c) Flame test : Clean the platinum wire Colour flame is not observed Zn+2 (or) Mn+2 , may be by dipping in con. HCl present and heat it on flame upto no colour appears. Now, prepare the paste of con.HCl + salt in watch glass, then introduce this paste by platinum wire loop into the flame. d) Action of heat : A small quantity of salt is Water droplets are formed on the May be Hydrated salt taken in a dry test tube and inner walls of test tube. strongly heated. II. Identification of Anion : a) Action of dil HCl : A small quantity of a salt is No reaction. Carbonate (CO–2 3 ) & aken in a dry test tube and Acetate (CH3 COO– ) ions dil.HCl is added. ions are absent. b) Action of Conc.H2 SO4 : A small quantity of salt is a) Before heating no reaction Chloride (Cl) & Bromide taken in a dry test tube and (Br– ) ions are absent. Conc.H2 SO4 is added. b) After heating no Reaction Nitrate (NO– 3 ) ion is absent c) Action of Cu turnings and Conc. H2 SO2 : To the small quantity of No reaction Nitrate (NO– 3 ) ion is absent. salt is taken in a test tube 1 or 2 pieces of Cu turning and conc. H2 SO4 are added and then heated. d) Action of BaCl solution : To the salt solution BaCl2 White ppt. of (BaSO4 ) is Sulphate (SO–2 4 ) ion is solution is added. formed. present III. Preparation sodium carbonate extract : To a small amount of the salt, add twice the amount of sodium carbonate, a few ml of distilled water and boil it for five minutes. It is filtered and the filtrate is known as sodium carbonate extract. IV. Confirmation test for Sulphate ion : Action of BaCl2 solution : To the salt solution BaCl2 White ppt. of (BaSO4 ) is formed. Sulphate (SO–2 4 ) ion is solution is added. Solubility: Above ppt. is insoluble confirmed. in conc.HCl.
- 42. Practical Chemistry eeee 58 eeee Janani Publications V. Identification of Cation : Preparation of original salt solution : Original salt solution can be prepared by dissolving excess amount of given salt in a suitable solvent. VI. Test for Ammonium (NH+ 4 )ion : Reaction with NaOH : To the small colour less gas (NH3 ) with Ammonium (NH+ 4 ) quantity of salt is taken in a dry test pungent smell is not evolved. cation is absent. tube few drops of NaOH solution is added and heated strongly. VII.Group Separation Table : a) Test for I Group Cations : To the salt solution a few drops of No ppt. is formed. Lead (Pb+2 ) cation is dil HCl solution is added. absent. b) Test for II Group Cations : To the salt solution a few drops of No ppt. is formed. Copper (Cu+2 ) cation dil HCl solution is added and is absent. H2 S gas is passed. c) Test for III Group Cations: To the salt solution NH4 Cl No ppt. is formed. Aluminium (Al+3 ) salt and NH4 OH solution are cation is absent. added. d) Test for IV Group Cations: Salt solution + NH4 Cl salt + NH4 OH solution + H2 S gas passed Flesh colour ppt. MnS is formed. Manganese (Mn+2 ) cation is present. e) Test for V Group Cations: Salt solution + NH4 Cl salt + White ppt. CaCO3 is not formed. Calcium (Ca+2 )absent NH4 OH solution +(NH4 )2 CO3 White ppt. BaCO3 is not formed. Barium (Ba+2 )absent solution is added White ppt. SrCO3 is not formed. Strontium (Sr+2 ) absent f) Test for VI Group Cations : Salt solution + NH4 Cl salt + White ppt. is not formed Magnesium (Mg+2 ) NH4 OH solution +(NH4 )2 CO3 + absent Na2 HPO4 solution is added VIII. Confirmation Tests for Magnesium : a)Action of NaOH solution : To the salt solution Sodium White ppt. Mn (OH)2 is formed. Manganes (Mn+2 ) Hydroxide solution (NaOH) Which turns to Brown colour. cation is is added. It is insoluble in excess of confirmed. NaOH solution. b) Action of NH4 OH solution : To the salt solution NH4 OH White ppt. Mn (OH)2 is formed. Manganese (Mn+2 ) solution is added. Which is soluble in excess of cation is confirmed. NH4 OH solution. IX. Report : The given Cation is : Manganese (Mg+2 ) The given Anion is : Sulphate (SO–2 4 ) The given salt is : Manganese Sulphate [MnSO4 ]
- 43. eeee 59 Practical Chemistry eeee Janani Publications SALT-22 -Nickel Nitrate . . EXPERIMENT OBSERVATION INFERENCE I. Priliminary Tests : : a) Colour : Pale pink Ni+2 cation may be present b) State : Crystalline c) Flame test : Clean the platinum wire Colour flame is not observed Ca+2 , Sr+2 , Ba+2 , Cu+2 , Zn+2 by dipping in con. HCl (or) Mn+2 , Pb+2 are absent. and heat it on flame upto no colour appears. Now, prepare the paste of con.HCl + salt in watch glass, then introduce this paste by platinum wire loop into the flame. d) Action of heat : A small quantity of salt is Reddish Brown vapours are May be Nitrate (NO3 – ) taken in a dry test tube and evolved salt is present strongly heated. II. Identification of Anion : a) Action of dil HCl : A small quantity of a salt is No reaction. Carbonate (CO–2 3 ) & taken in a dry test tube and Acetate (CH3 COO– ) dil.HCl is added. ions are absent. b) Action of Conc.H2 SO4 : A small quantity of salt is a) Before heating no Reaction Chloride (Cl) & Bromide taken in a dry test tube and (Br– ) ions are absent. conc.H2 SO4 is added. b) After heating Reddish Brown Nitrate (NO– 3 ) ion may be vapours are evolved present Action of Cu-turnings and Conc. H2 SO2 : To the small quantity of salt is Reddish Brown vapours of Nitrate (NO– 3 ) ion is taken in a test tube 1 or 2 NO2 are evolved and the present. pieces of Cu turning and solution turns pale blue Conc. H2 SO4 are added and then heated. III. Preparation sodium carbonate extract : To a small amount of the salt, add twice the amount of sodium carbonate, a few ml of distilled water and boil it for five minutes. It is filtered and the filtrate is known as sodium carbonate extract. IV. Confirmation test for Nitrate ion : a) Brown – Ring test : A small quantity of salt Dark Brown Ring is formed Nitrate (NO– 3 ) ion is solution is taken in a test tube in between the junction of confirmed. and same amount of freshly two layers. (Nitroso ferrous prepared FeSO4 solution is sulphate) (FeSO4 .NO) added, then few drops of conc.H2 SO4 is added by keeping the test tube in an inclined position.
- 44. Practical Chemistry eeee 60 eeee Janani Publications V. Identification of Cation : Preparation of original salt solution : Original salt solution can be prepared by dissolving excess amount of given salt in a suitable solvent. VI. Test for Ammonium (NH+ 4 )ion : Reaction with NaOH : To the small colour less gas (NH3 ) with Ammonium (NH+ 4 ) quantity of salt is taken in a dry test pungent smell is not evolved. cation is absent. tube few drops of NaOH solution is added and heated strongly. VII.Group Separation Table : a) Test for I Group Cations : To the salt solution a few drops of No ppt. is formed. Lead (Pb+2 ) cation is dil HCl solution is added. absent. b) Test for II Group Cations : To the salt solution a few drops of No ppt. is formed. Copper (Cu+2 ) cation dil HCl solution is added and is absent. H2 S gas is passed. c) Test for III Group Cations: To the salt solution NH4 Cl No ppt. is formed. Aluminium (Al+3 ) salt and NH4 OH solution are cation is absent. added. d) Test for IV Group Cations: Salt solution + NH4 Cl salt + NH4 OH solution + H2 S gas passed Black colour ppt. Nis is formed. Nickel (Ni+2 ) cation is present. e) Test for V Group Cations: Salt solution + NH4 Cl salt + White ppt. CaCO3 is not formed. Calcium (Ca+2 )absent NH4 OH solution +(NH4 )2 CO3 White ppt. BaCO3 is not formed. Barium (Ba+2 )absent solution is added White ppt. SrCO3 is not formed. Strontium (Sr+2 )absent f) Test for VI Group Cations : Salt solution + NH4 Cl salt + White ppt. is not formed Magnesium (Mg+2 ) NH4 OH solution +(NH4 )2 CO3 + absent Na2 HPO4 solution is added VIII. Confirmation Tests for Nickel : a) Action of NaOH solution : To the salt solution sodium Green gelatinous ppt.Ni(OH)2 is Nickel (Ni+2 ) cation Hydroxide solution (NaOH) formed. It is soluble in excess of is confirmed. is added. NaOH solution b) Action of NH4 OH solution : To the salt solution NH4 OH Green ppt. Ni (OH)2 is formed. Nickel (Ni+2 )cation solution is added. It becomes blue violet on addition is confirmed. of excess of NH4 OH solution IX) Report: The given Cation is : Nickel (Ni+2 ) The given anion is : Nitrate (NO– 3 ) The given salt : Nickel Nitrate [Ni(NO3 )2 ]
- 45. eeee 61 Practical Chemistry eeee Janani Publications EXPERIMENT OBSERVATION INFERENCE I. Priliminary Tests : : a) Colour : White Cu+2 , Fe+2 , Ni+2 & Mn+2 Cations may be absent b) State : Crystalline c) Flame test : Clean the platinum wire by Crimson red colour flame is Sr+2 may be present dipping in con. HCl and observed. heat it on flame upto no colour appears. Now, prepare the paste of con.HCl + salt in watch glass, then introduce this paste by platinum wire loop into the flame. d) Action of heat : A small quantity of salt is Water droplets are formed on the May be Hydrated salt taken in a dry test tube inner walls of test tube. and strongly heated. II. Identification of Anion : a) Action of dil HCl : A small quantity of a salt No reaction. Carbonate (CO–2 3 ) & is taken in a dry test tube Acetate (CH3 COO– ) ions and dil.HCl is added. ions are absent. b) Action of Conc.H2 SO4 : A small quantity of salt is Colour less gas (HCl) with Chloride (Cl– ) ion taken in a dry test tube and pungent smell is evolved and may be present. Conc.H2 SO4 is added. gives dense white fume (NH4 Cl) with glass rod when dipped in NH4 OH. III. Preparation sodium carbonate extract : To a small amount of the salt, add twice the amount of sodium carbonate, a few ml of distilled water and boil it for five minutes. It is filtered and the filtrate is known as sodium carbonate extract. IV. Confirmation test for Chloride ion : a) Action of MnO2 & Conc. H2 SO4 : To the small quantity of salt is Greenish yellow colour Chloride (Cl– ) ion is taken in a test tube pinch of MnO2 vapours (Cl2 ) with pungent confirmed. and Conc. H2 SO4 are added. smell are evolved. b) Action of AgNO3 solution : To the salt solution AgNO3 white curdy ppt. of (AgCl) is formed. Chloride (Cl– ) ion solution is added. Solubility: Above ppt. is is confirmed completely soluble in NH4 OH V. Identification of Cation : Preparation of original salt solution : Original salt solution can be prepared by dissolving excess amount of given salt in a suitable solvent. SALT-23 -Strontium Chloride . .
- 46. Practical Chemistry eeee 62 eeee Janani Publications VI. Test for Ammonium (NH+ 4 )ion : Reaction with NaOH : To the small colour less gas (NH3 ) with Ammonium (NH+ 4 ) quantity of salt is taken in a dry test pungent smell is not evolved. cation is absent. tube few drops of NaOH solution is added and heated strongly. VII. Group Separation Table : a) Test for I Group Cations : To the salt solution a few drops of No ppt. is formed. Lead (Pb+2 ) cation is dil HCl solution is added. absent. b) Test for II Group Cations : To the salt solution a few drops of No ppt. is formed. Copper (Cu+2 ) cation dil HCl solution is added and is absent. H2 S gas is passed. c) Test for III Group Cations: To the salt solution NH4 Cl No ppt. is formed. Aluminium (Al+3 ) salt and NH4 OH solution are cation is absent. added. d) Test for IV Group Cations: Salt solution + NH4 Cl salt + White ppt. ZnS is not formed. Zinc (Zn+2 ) absent NH4 OH solution + H2 S gas passed Flesh colour ppt. MnS is not formed. Manganese (Mn+2 ) absent Black ppt. Nis is not formed. Nickel (Ni+2 ) absent e) Test for V Group Cations: Salt solution + NH4 Cl salt + Calcium (Ca+2 ) NH4 OH solution +(NH4 )2 CO3 Barium (Ba+2 ) solution is added White ppt. is formed. Strontium (Sr+2 ) cations are present f) Test for VI Group Cations : Salt solution + NH4 Cl salt + White ppt. is not formed Magnesium (Mg+2 ) NH4 OH solution +(NH4 )2 CO3 + absent Na2 HPO4 solution is added VIII. Confirmation Tests for Strontium : a) Action of K2 CrO4 solution : To the salt solution sodium Yellow ppt. (SrCrO4 ) is formed. Strontium (Sr+2 )cation Potassium chromate solution It is soluble in acetic acid. is confirmed. (K2 CrO4 ) is added. NaOH solution b) Action of Ammonium Sulphate solution: To the salt solution White ppt. is formed. Strontium (Sr+2 ) cation Ammonium Sulphate It is sparingly soluble in is confirmed. solution is added. acetic acid and highly soluble in dil.HCl IX) Report: The given Cation is : Strontium (Sr+2 ) The given anion is : Chloride (Cl– 3 ) The given salt : Strontium chloride [SrCl2 ]
- 47. eeee 63 Practical Chemistry eeee Janani Publications SALT-24 -Zinc Sulphate . . EXPERIMENT OBSERVATION INFERENCE I. Priliminary Tests : : a) Colour : White Cu+2 , Fe+2 , Ni+2 & Mn+2 Cations may be absent b) State : Crystalline c) Flame test : Clean the platinum wire by Green flashes colour flame is Zn+2 or Mn+2 may be dipping in con. HCl and observed. present heat it on flame upto no colour appears. Now, prepare the paste of con.HCl + salt in watch glass, then introduce this paste by platinum wire loop into the flame. d) Action of heat : A small quantity of salt is Water droplets are formed on the May be Hydrated salt taken in a dry test tube inner walls of test tube. and strongly heated. II. Identification of Anion : a) Action of dil HCl : A small quantity of a salt is No reaction Carbonate (CO–2 3 ) & taken in a dry test tube and Acetate (CH3 COO– ) dil.HCl is added. ions are absent. b) Action of Conc.H2 SO4 : A small quantity of salt is a) Before heating no reaction Chloride (Cl– ) & taken in a dry test tube and Bromide (Br– )ions are absent. Conc.H2 SO4 are added. b) After heating no reaction Nitrate (NO– 3 ) ion is absent. c) Action of Cu turnings and Conc. H2 SO4 : To the small quantity of No reaction Nitrate (NO– 3 ) ion is absent salt is then taken in a test tube 1 or 2 pieces of Cu turnings and Conc.H2 SO4 are added and then heated d) Action of BaCl2 solution : To the salt solution BaCl2 White ppt. of (BaSO4 ) is formed. Sulphate (SO–2 4 ) ion is olution is added. present. III. Preparation sodium carbonate extract : To a small amount of the salt, add twice the amount of sodium carbonate, a few ml of distilled water and boil it for five minutes. It is filtered and the filtrate is known as sodium carbonate extract.
- 48. Practical Chemistry eeee 64 eeee Janani Publications IV. Confirmation test for Sulphate ion : Action of BaCl2 soution : To the salt solution BaCl2 White ppt. of (BaSO4 ) is Sulphate (SO–2 4 ) ion is solution is added. formed. confirmed. Solubility : Above ppt. is insoluble in Conc. HCl V. Test for Ammonium (NH+ 4 )ion : Reaction with NaOH : To the small colour less gas (NH3 ) with Ammonium (NH+ 4 ) quantity of salt is taken in a dry test pungent smell is not evolved. cation is absent. tube few drops of NaOH solution is added and heated strongly. VI. Group Separation Table : a) Test for I Group Cations : To the salt solution a few drops of No ppt. is formed. Lead (Pb+2 ) cation is dil HCl solution is added. absent. b) Test for II Group Cations : To the salt solution a few drops of No ppt. is formed. Copper (Cu+2 ) cation dil HCl solution is added and is absent. H2 S gas is passed. c) Test for III Group Cations: To the salt solution NH4 Cl No ppt. is formed. Aluminium (Al+3 ) salt and NH4 OH solution are cation is absent. added. d) Test for IV Group Cations: Salt solution + NH4 Cl salt + White ppt. ZnS is formed. Zinc (Zn+2 ) cation NH4 OH solution + H2 S gas passed is present e) Test for V Group Cations: Salt solution + NH4 Cl salt + White ppt. CaCO3 is not formed. Calcium (Ca+2 ) absen NH4 OH solution +(NH4 )2 CO3 White ppt. BaCO3 is not formed. Barium (Ba+2 ) absent solution is added White ppt. SrCO3 is not formed. Strontium (Sr+2 ) absent f) Test for VI Group Cations : Salt solution + NH4 Cl salt + White ppt. is not formed Magnesium (Mg+2 ) NH4 OH solution +(NH4 )2 CO3 + absent Na2 HPO4 solution is added VII. Confirmation Tests for Zinc : a) Action of NaOH solution : To the salt solution sodium White ppt. Zn (OH)2 is formed. Zinc (Zn+2 ) cation is Hydroxide solution (NaOH) Above ppt. is soluble in excess of confirmed is added. NaOH solution. b) Action of NH4 OH solution: To the salt solution NH4 OH White Gelatinous ppt. is Zinc(Zn+2 ) cation is solution is added. formed Above ppt. is soluble in is confirmed. excess of NH4 OH solution VII. Report : The given Cation is : Zinc (Zn+2 ) The given anion is : Sulphate (SO–2 4 ) The given salt : Zinc sulphate [ZnSO4 ]