More Related Content
Similar to Lipid Photosynthesis Electron Transport Chain
Similar to Lipid Photosynthesis Electron Transport Chain (20)
Lipid Photosynthesis Electron Transport Chain
- 1. 2H+
H+
OXALOAC E TATE
PYR UVATE
S UC C INYL-C oA
GLUTAR ATE
C ITR ATE
MALATE
2-OXO- AS PAR TATE
NO2
-
NO3
-
N2
NH4
CH3COSCoA
ACE TYL-CoA
HOH
CO2
NAD
TRANSA
MINATION
2H+
2H
+
+
CO2
5-Amino-
levulinate
Glycine
H+
H+
H+
H+
H+
H+
c
H+ H+a
F0
F1
F1
β2
1 α
β
α
γ
10 c-sub-units
AD
P+ Pi
H+
3.6.1.34
H
+-transporting ATP synth
ase
α
δ
F6
oscp
ATP
ADP +Pi
FUMAR ATE
UQH2
UQ
UQH2
NADH+H+
CH2COO-
C(OH)COO-
CH2COO-
CH(OH)COO-
CHCOO-
CH2COO-
-OOCCOCH2CH2COO-
-OOCCH2CH2CO.SCoA
-OOCCOCH2COO-
CH3CH(OH)CH2CO.SCoA
-OOCCH=CHCOO-
Glyoxylate
Cycle
-OOCCHO
GTP
GDPATP
+
HE ME Protoporphyrinogen Coproporphyrinogen Uroporphyrinogen Porphobilinogen
5-Amino-
levulinate
COO-
CH2
CH2
H2NCH2C=O
FADH2
FADCyt.b
Fe-S
S UC C INATE
II
CH3COCOO-
PYR UVATE
Pi
X Y
Cyt.c1
Fe-S
2e-
2e-
1e-
1e-
III
2UQ UQ
UQH22UQH2
2UQ.
_
UQ.
_
Cyt.bL Cyt.bH
Cyt.c
IV
CuA
CuB
CuA
1.9.3.1
2e-
Heme a
Heme a3
2H+
2H+4H+
4H+
2H+
NAD+
NADH+H+
NAD+
1.1.1.39
1.2.4.1
2.3.1.12
3.1.3.43
4.1.1.32
4.1.3.7
4.2.1.3
1.1.1.41
1.2.4.2
2.3.1.61
6.2.1.4
-OOCCH2CH2COO-
1.3.5.1
4.2.1.2
1.1.1.37
6.4.1.1
4.1.3.8
4.1.3.1
4.1.3.2
IS OC ITR ATE
2.6.1.1
2.3.1.16
4.1.1.71
1.2.1.16
5.1.99.1
5.4.99.2
4.3.1.1
1.4.1.2 1.4.1.14
6.3.5.4
1.6.6.1
1.7.99.4 1.7.7.1
1.6.6.4
1.18.6.1
2.3.1.37
1.10.2.2
1.10.2.2
γ
εδ
β
33
β
AT
P
ADP
E NDE R G ONIC R E AC TION
1.6.5.3
I
FMNH2
FMN I
1.6. 5.3
2Fe -S
(5 Clusters)
H+ H+
H+ H+
ATP
4H+
2H+
2H+
or
H+
PHOTO-
SYSTEM
l
O2
3.6.1.34
NADP+
Glyceraldehyde
Ribulose-1,5-bis-P
2β
88
H+
c
32
ε
a
32
α1
α
ε
α
β2
1
3
α
α
β
3
α
β
H+
H+
H+
H+
H+H+
H+
H+
H+H+
H+H+H+H+
H+
H+
H+H+
H+
H+
H+
H+
H+
H+
H+
H+
H+
H+H+
H+H+
H+H+H+
Translocated protons
H+
H+
H+
H+
Pi
Ferredoxin
4H+
PC PC
PC
PQH2
2PQH2
PQ
_
.
2PQ
_
.
Fe-S Cyt.f
PQ
2PQ
Cyt bf
2e-
1e- 1e-
2e-
2e-
2e-
2e-
Cyclic Photophosphorylation
PQ
PHOTO-
SYSTEM
II
Cyt bc
Non-cyclic electron flo
w
2e-
2e-
Mn
2e-
*
Protons from Water
H2O
H+
H+
2H+
2H+
γ
ATP synthase
CO2
Fixation
H+
ADPPi
H+
NADPH+H+
ATPTHYLAKOID MEMBRANE
CHLOROPLAST OUTER MEMBRANE
THYLAKOID LUMEN
STROMA
(electric current)
P680
Chl.a
QA
QB
Pheophytin
P700
Chl.A0
A1
Fe-S
ATP
ADP
ATP
1/2O2
H2O
PQH2
To B rain -
A
M
I
N
O
A
C
I
D
S
P
Y
R
I
M
I
D
I
N
E
S
P
U
R
I
N
E
S
C
A
T
E
C
H
O
L
A
M
I
N
E
S
A
R
O
M
A
T
I
C
A
M
I
N
O
A
C
I
D
S
hv
VIS ION
L
I
P
I
D
P
H
O
T
O
S
Y
N
T
H
E
S
I
S
P
O
R
P
H
Y
R
I
N
S
S
T
E
R
O
I
D
S
I
S
O
P
R
E
N
O
I
D
S
P
H
O
S
P
H
O
L
I
P
I
D
S
D
E
G
R
A
D
A
T
I
O
N
L
I
P
I
D
B
I
O
S
Y
N
T
H
E
S
I
S
P
E
N
T
O
S
E
S
H
E
X
O
S
E
S
P
O
L
Y
S
A
C
C
H
A
R
I
D
E
S
CO CH CH(NH )COO2 3
+
FOLIC
ACID
C1
POOL
VALINE
ATP
ATP
4.1.1.4 1.1.1.30
CH COCH3 3
CH COCH COO3 2
CH CH(OH)CH COO3 2
3-OH-B utyrateAcetone
Acetoacetate
K E TONE B ODIE S
HYALUR ONIC ACID DE R MATAN B LOOD GR OUP
S UB S TANCE S
PE PTIDO-
GLYCAN
N-Ac-Neuraminate
(S ialate)
UDP-
Iduronate
UDP-N-Ac-
Galactos amine
UDP-
Galacturonate
GDP-Fucos e
TDP-R hamnos e
ADP-
Glucos e
UDP-Glucos e
TDP-4-Oxo-
6-deoxyglucos e
MANNOS E
CHITIN CHONDR OITIN PE CTIN
ALGINATE S
INULIN CE LLULOS E
O-ANTIGE NS S TAR CH GLYCOGE N
LACTOS E
GALACTOS E
O
OH
HO
OH
OH
CH3 O
OPPT
O
OH
OH
O
OH
ACNH
HO OH
O
OHHO OPPU
NHAC
OH
OH OH
OH
HO
OH
NHCOCH
OH HO
O
OPPG
CH OH2
HO
P
UDP-N-Ac-
Glucos amine
pyruvate
N-Ac-Mannos amine-6-P UDP-Glucuronate
GDP-Mannos e
N-Ac-Mannos amine UDP-N-Ac-Glucos amine N-Ac-Glucos amine-6-P
S orbitol
Fructos e-1-P
E rythros e-4-P
3-Dehydrogulonate
L-Xylos e
L-Lyxos e
D-Xylulos e
D-R ibos e
R ibitol
L-Arabitol L-Xylulos e
L-R ibulos e L-R ibulos e-5-P
L-Xylulos e-5-P
L-Arabinos e Xylitol D-Xylos e
D-Arabinos e D-R ibulos e
AS COR B ATE
2, 3-Dioxogulonate
N-Ac-Glucos amine-1-P
Inos itol-PInos itolGlucuronateGulonate
Gulonolactone 2-Oxogulonolactone
Fructos e
6-P-Gluconate
D-R ibulos e-5-P
D-Xylulos e-5-P
Mannos e-1-P
GDP-Glucos e
TDP-Glucos e
UDP-Galactos e
Galactos e-P
Glucos e-1-P
Glucos e-6-P
Fructos e-6-P
Fructos e-
1:6-bis -P
OH
H H H
O
HOCH2 C C C C CO
OH OHH OH
H H
O
HOCH2 C C C CO CO
OHH
H
OH H
C C
OH
CO
P-R ibos yl
amine
Glycerate
2, 3-Diphos pho-
glycerate
3-P-Glyceraldehyde
1:3-bis -P-Glycerate
3-P-Glycerate
2-P-Glycerate
P-enolpyruvate
Inositol
CH OH2
CH OH2
HOCH
CH OH2
CH O2
HOCH
P
Glycerol
UDP-Galactos e
UDP-S ugars
CH CH OH3 2
E THANOL
Dihydroxy-
acetone-P
(Glycerone-P)
Glucos amine-6-P
Phos pho-
s erine
Chain elongation Mitochondrial
S erine
S erine
Indole-3-glycerol-P
3-Deoxy-D-arabino-
heptulos onate-7-P
Indoleacetate
(Auxin)
Indoxyl
Formylkynurenine K ynurenine 3-Hydroxy
kynurenine
3-Hydroxy
anthranilate
2-Amino-3-carboxy
muconate s emialdehyde
NH
OH
Tryptamine 1-(o-Carboxy phenylamino)
1-deoxyribulos e-5-P
N-(5-P-R ibos yl)
anthranilate
Anthranilate
Catechol
NH
Indole-
acetaldehyde
NH
CH CHO2
Indolepyruvate
N
H
C-CH(OH)CH(OH)CH O2 P
CH
2-Aminomuconate-
6-s emialdehyde
CH CH NH2 2 2
NH
1.2.3.7
4.1.1.43
3.5.1.9 3.7.1.3
4.2.1.20
1.13.11.6 4.1.1.45
2.4.2.18
4.6.1.4
4.1.1.48
1.14.13.9
4.6.1.3
Dehydro-
quinate
4.2.1.10 1.1.1.25 2.5.1.19
S hikimate-3-P S hikimate-5
enolpyruvate 3-P
Choris mate
PE P
2.7.1.71
1.4.1.19
Homogentis ate Phenylpyruvate
Cinnamate
Coumarate
Prephenate
2-Amino
muconate
Fumarate As partate
GLYCINE
S arcos ine
Hydroxy-
pyruvate
P-Hydroxy-
pyruvate
S E R INE
Imidazole
glycerol-P
P-R ibos yl-ATP P-R ibos ylformimino
5-aminoimidazole-
carboxamide-R P
Formimino
glutamate
Imidazolone
propionate
Urocanate
Cys tathionine Homocys teine
Phos phoadenylyl-
s ulphate
(PAPS )
CH (NH )COOH2 3
+
His tidinol-PHis tidinolHis tidinal
3.1.3.15 2.6.1.91.1.1.23
3.6.1.31
5.3.1.162.4.2.17
As paragine
ALANINE
3-S ulphinyl
pyruvate
Oxobutyrate
2-Aceto-2-
hydroxy-
butyrate
O-Phos pho-
homos erine
HO S CH CH NH2 2 2 2
Hypotaurine
HO S CH CH NH3 2 2 2
Taurine
Glutamate
γ-Glutamylcys teine
Glutathione
Glycine
Cys teine
ß-Alanine
Cys teate
S -Adenos yl
homocys teine
4.2.99.2
1.1.1.86
4.2.1.9
1.1.1.86 4.2.1.9
2.6.1.32
4.1.3.18
S -Adenos yl
methionine
(S AM)
2-Methylaceto-
acetyl-CoA
2-Acetolactate 2-3-Dihydroxy
is ovalerate
2-Oxo-
is ovalerate
3-Hydroxy-
is obutyrate
3-Hydroxy-
Is obutyryl-CoA
Methyl
acrylyl-CoA
2-Methyl-3-
hydroxy-
butyryl-CoA
Tiglyl-CoA 2 Methylbutyryl-
CoA
2:3-Di-OH-
3-methylvalerate
2-Oxo-3-methyl
valerate
Is obutyryl-CoA
Cys teine
s ulphinate
4.2.99.9
2-Is opropyl-
malate
CH CH COCOO3 2
+
CH CH(NH )COO3 3
CH CH(OH)COO3
HO S CH COCOO2 2
OCH COCOO2P
OCH CH(NH )COO2 3P
HOCH COCOO2
HOCH CH(NH )COO2 3
+
+
OH
CH COCOO2
COO
OH
OH
O
COO
OH
OH
HO P
COO
OH
OH
O
CH2
P
COO
O-C-COO
OH
O
OH
OOC CH COCOO2
N
H
HOC-CH(OH)CH(OH)CH 2OP
CH
COO
NH2
COO
OH
NH2COO
COO
OCH
OOCCH NHCH2 3
CH (CH ) COS -ACP3 2 14 CH (CH ) COS CoA3 2 14
CH (CH ) CH=CHCO-S -ACP3 2 2
CH CH=CHCO-S -ACP3 CH CH(OH)CH COS -ACP3 2
COS CoA
Linoleate
Oleoyl-CoA
S tearoyl-CoA Dehydros tearoyl-CoA OH-S tearoyl-CoA Oxos tearoyl-CoA
Palmitoyl-ACP Palmitoyl-CoA
ACYL-ACP 2, 3-E noyl-ACP
3, 4-Decenoyl-ACP
2, 3-Decenoyl-ACP
3-OH-Acyl-ACP 3-Oxoacyl-ACP
3-Oxo-Decanoyl-ACP
3-Oxo-Hexanoyl-ACP3-OH-Hexanoyl-ACP2, 3-Hexenoyl-ACP
B utanoyl-ACP Crotonoyl-ACP 3-OH-B utanoyl-ACP Acetoacetyl-ACP
Hexanoyl-ACP
3-OH-Decanoyl-ACPDecanoyl-ACP
Palmitoleoyl-ACP
γ-Linolenate Arachidonate Leukotriene B 4
COS CoA
CO-S -ACP
Thromboxane B 2
HOOCCH CO-S -ACP2
Malonyl-ACP
HOOCCH CO-S CoA2
Malonyl-Co-A
CH CO-S -ACP3
Acetyl-ACP
CH O-CO-R2
CH OH2
R ’-CO-OCH
FATTY ACID
ACYL-CoA
(Cytos ol)
CH O-CO-R2
CH O2
R ’-CO-OCH
P
Cardiolipin Phos phatidylglycerol
Acetylcholine
Glycerophos phocholine
Lys olecithin
Choline
plas malogen
CDP-choline Choline-P
Mevaldate
CH (CH ) CH=CHCH(OH)CHCH OH3 2 12 2
Dehydros phinganin S phinganin 4-S phingenin Ps ychos ine
Acyl-CoA
Acyl-CoA
Cerebros ide
Galactos eCH (CH ) CH=CHCH(OH)CHCH O-3 2 12 2
NHCOR
Is opentenyl-PP
(C5)
Dimethylallyl-PP
(C5)
Geranyl-geranyl-PP
(C20)
Farnes yl-PP
(C15)
S qualene
(C30)
Geranyl-PP
(C10)
Des mos terol Zymos terol Lanos terol
CH C = CHCH O3 2 PP
CH3
Ceramide
Ganglios ides
NHCOR
CH O2 PP
OPP
CH2
CHOLE S TE R OL
CH C(OH)CH CH OH3 2 2
CH COO2
CH C(OH)CH CHO3 2
CH COO2
NADP +
Dehydroas corbate
OH
H O
HOCH2 C C C C CO
OHH OH
OH
H O
HOCH2 C C CO CO CO
H
NAD+
Pi
ADPADP
Phytoene
(C40)
Lycopene (C40)
ß-CAR OTE NE (C40)
R hodops inMetarhodops in
R etinoate
trans -R etinal
11-cis -R etinoltrans -R etinol
(Vitamin A)
R etinol es ters
Dark
Light
Ubiquinone
(Coenzyme Q)
Menaquinone
Plas toquinone
Phylloquinone
(Vitamin K )
Ops in
CHO
CH OH2
CHO
CH OH2
CH3CH O3
CH O3 n
O
O
O
O
Phytol (C20)
α-Tocopherol
(Vitamin E )
Quinolinate
Quinolinate-
nucleotide
Nicotinate-
nucleotide
Des amino-NAD
5-Hydroxy-
tryptophan
5-Hydroxytryptamine
(S E R OTONIN)
N-Acetyl-5-O-methyl-s erotonin
(ME LATONIN)
NH
CH CH NHCOCH2 2 3HO
NH
CH CH NHCOCH2 2 3CH O3
+
NAD( P)
NICOTINATE
N-Acetyl-s erotonin
2.4.2.19
Dopamine Dopa
Dopaquinone
THYR OXINEME LANIN
OH
OH
CHOHCH NHCH2 3
OH
OH
CHOHCH NH2 2
4-OH-3-Methoxy-
phenylglycol
CHOHCH NH2 2
OH
OCH3OCH3
CHOHCH OH2
OH
OCH3
4-OH-3-Methoxy-
D-mandelate
Cyclic AMP
ATP
Dephos pho-Coenzyme A
4-P-Pantetheine
4-P-Pantothenylcys teine
4-P-Pantothenate
Pantoate
6.3.2.1
1.1.1.169
3.5.1.22
2.7.1.33
NH
NH
O
O
O
O
CH OH2
OH
OCH3
CH(OH)COO
H2
NH3
+
CH-COO
CO
O
COO
NR ibose- P
N
+
COO
NH
CH COO2
O
OH
CH O2 P
NHCOCH NH2 2
OH
Formyl
glycinamide-R P
Urea
Formyl
glycinamidine-R P
Allantoate Allantoin UR ATE
ADP
Xanthine Hypoxanthine
Inos ine
Adenine
Adenylo-
s uccinate
5-Amino
imidazole-R P
5-Amino-4-imidazole
carboxylate-R P
5-Amino-4-imidazole
(N-s uccinylcarboxamide)-R P
5-Aminoimidazole
carboxamide-R P
Formylamido-
imidazole-
carboxamide-R P
H NCONH2 2
HN
H C2 CHO
NH
R P
NH
C
C
C
O
C
CO
OC
NH
NH
H
N
HN
H
C
C
O
C
CH
OC
NH
N
N
HN
Carbamoyl
ß-alanine
Dihydro-
uracil Uracil
d-ADP
d-ATP
GTP GDP
XANTHOS INE -P
(XMP)
TTP
TDP
ß-Ureido
is obutyrate
Dihydro
thymine
Thymine d-UMP d-CMP d-CDP
CDP
Carbamoyl
as partate
Dihydro
orotate
Orotate Orotidine-P Uridine-P
(UMP)
UDP
3-Amino-
is obutyrate
CH
C
NH2
CHOC
NH
NCH
C
O
CHOC
N H
HN
CH-CH3
C
O
OC
NH
HN
CH2
CH2
CH2
C
O
OC
NH
HN
C
O
CHOC
N H
HN C CH3
Methylmalonyl
s emialdehyde
OHCCHCOO
CH3
CH2
C
O
CH-COOOC
NH
HN CH
C
O
C-COOOC
N H
HN
H NCONHCH CH COO2 2 2
C
C
C
CH
R P
HC
NH
NH
OOC-CH-CH COO2
N
N
N
CHO
NH
R P
NH
OC
H C2
2.7.7.43
3.1.3.29
1.1.1.158
5.1.3.13
2.7.1.38
3.2.1.23
3.1.3.29
4.1.3.20
2.7.1.60
5.1.3.14
5.1.3.7
5.1.3.1
2.7.1.4
5.4.2.3
3.1.1.17
1.1.1.49
1.1.1.21
2.7.7.23
3.1.3.251.13.99.11.1.1.19
1.1.3.8
5.3.1.3
2.7.1.53
1.1.1.9
2.7.1.15
2.7.1.17
5.1.3.4
5.1.3.1
2.2.1.2
4.1.2.13
2.7.1.11
2.7.1.47
2.2.1.1
1.2.1.12
1.1.1.29
1.1.1.95
1.3.1.35
1.14.99.25
2.3.1.41
2.3.1.41
2.3.1.41
1.1.1.100
5.3.99.5
4.2.1.60
5.3.99.3
1.14.99.1
1.13.11.34
1.3.1.10
1.3.1.9
1.14.99.5
1.3.1.9
4.2.1.61
4.2.1.60
4.2.1.60
4.2.1.59
4.2.1.58
1.3.1.9
1.3.1.9
6.2.1.3 3.1.2.20
1.1.1.100
5.3.1.1
HOOC-COOH
Oxalate
Glycolate
HOCH CHO2
Glycol
aldehyde
E thanolamine-P
2.7.1.30
2.3.1.7
3.7.1.2
4.1.3.5
2.7.8.8
2.1.1.17
2.1.1.71
1.3.1.35 2.7.8.2
3.1.4.3
3.1.4.4
2.7.7.15
3.1.1.5 3.1.4.2
2.7.1.32
2.7.1.82
1.2.4.1
2.3.1.12
1.8.1.4
2.6.1.2
1.4.1.1
4.1.1.1
2.3.1.50
1.1.1.102
3.1.4.12
2.7.8.3
2.4.1.62
3.2.1.46
5.2.1.3
1.2.1.36
2.3.1.76
3.1.1.21
5.2.1.7
5.4.99.7
1.14.99.7
4.3.1.8
4.2.1.75
4.1.1.371.3.3.31.3.3.4
4.99.1.1
2.5.1.29
2.4.1.47
4.1.1.28 2.3.1.5 2.1.1.4
6.3.5.1
6.3.1.5
2.4.2.11
2.7.7.18
2.6.1.5
1.2.1.32
1.13.11.5
2.1.1.28
2.1.2.2 6.3.3.1
3.5.2.5
1.4.1.10
1.7.3.3 1.1.1.204 1.1.3.22
1.1.3.22
4.1.1.28
4.1.1.21
2.1.2.3
2.4.2.1
1.5.99.2
2.1.1.5
2.6.1.22
3.5.2.3
2.6.1.51
1.4.1.7
3.1.3.3
2.6.1.52
2.1.3.2
ACE TATE
2.4.2.4
2.1.1.45
R P
CH
C
O
CHOC N
HN
1.3.1.14 2.4.2.10 4.1.1.23
2.7.7.7
2.7.7.7
2.7.7.7
1.17.4.1
2.7.7.7
2.7.4.14
4.4.1.1
1.6.4.1
1.13.11.20
4.4.1.8
4.2.1.22
1.1.1.27
4.1.1.29
6.3.2.3
6.3.2.2
1.8.1.3
LACTATE
Acetaldehyde
2.7.1.24
2.7.7.3
4.1.1.36
B ile Acids
CH CH COS CoA3 2
Propanoyl-CoA
1.1.1.31
2.1.3.1
4.1.1.41
5.1.99.1
6.4.1.3
6.3.2.5
2.1.1.14
2.1.1.13
4.6.1.1
CDP-E thanolamine
1.1.1.35 4.2.1.17
+
HO S CH CH(NH )COO2 2 3
3.5.2.7
NHHN
CH
OOC CHCH CH COO2 2
CMP-N-Acetyl
neuraminate
CDP-diacyl
glycerol
CHOLINE
E thanolamine
Glyoxylate
HIS TAMINE
2.3.1.46
Homos erine
Tyramine
Plant PigmentsTannins
Maleyl
acetoacetate
Fumaryl
acetoacetate
5.2.1.2
CH COO2
OH
OH
OH
CH CH NH2 2 2
OH
OH
CH CH NH2 2 2
Hydroxyphenyl
pyruvate
α-Tocopherol
(Vitamin E )
LIGNIN
d-GTP
OH
OH OH
O
HO
OH
P
OPPT
CH3
HO
OHOH
O
NADPH
2.2.1.1
2.7.1.3
1.1.1.45
1.1.1.130
1.10.3.3
1.10.2.1
1.1.1.10
ATP
CH CH NH2 2 2
NH
HO
5.4.99.5
1.14.16.12.6.1.51.13.11.27
Indole
4.1.99.1
1.13.11.11
OH
O
OH
NH C C CH CH O2 P
H HCOO
NH2
COO
6.3.5.3 6.3.2.6 4.3.2.2
Guanine
DNA
6.3.4.44.3.2.2
C
C
O
C
CH
HC
NH
N
N
HN C
C
O
C
CH
HC
NH
N
N
HN
3.5.3.4
2.4.2.1
2.7.7.62.7.7.6
2.7.7.6
2.7.7.6
2.7.4.6
2.7.4.3
2.7.4.4
2.7.4.6
2.4.2.15
1.1.1.205
6.3.4.1
6.3.5.2
2.7.4.4
3.5.4.3
3.2.2.2
2.4.2.1
3.1.3.5
3.1.4.6
6.3.4.2
1.17.4.1
1.17.4.1
3.5.4.12
2.7.4.8
2.7.4.9
2.7.4.6
2.4.2.4
1.3.1.2
3.5.2.23.5.1.6
3.5.1.6 3.5.2.2 3.5.4.1
Diphos pho-
mevalonate
Mevalonate
3-Oxopentanoyl-CoA
3-Oxoacyl-CoA3-OH-Acyl-CoA2, 3-E noyl-CoA
2, 3-Hexenoyl-CoA 3-OH-Hexanoyl-CoA
Acetoacetyl-CoA3-OH-B utanoyl-CoACrotonoyl-CoA
Pentanoyl-CoA 3-OH-Pentanoyl-CoA
1.1.1.35
1.1.1.35
1.1.1.157
1.3.99.3
1.3.99.3
1.3.99.2
1.1.1.35
Odd C Fatty acids
S uccinylhomos erine
CO2
2-OXO ACID
Glutamyl-P
Glutamine
UR E A
P-CreatineCreatine Creatinine
Glycine
CITR ULLINE
Glutamic
s emialdehyde
2-AMINO ACID
Pyrroline-5-
carboxylate
GlyoxylatePyruvate
4-Hydroxy-
2-oxoglutarate
4-Hydroxy-
glutamate
3-Hydroxy-
pyrroline-
5-carboxylate
Putres cine
H NCH CH CH CH NH2 2 2 2 2 2
S permidine
S permine
N -Trimethyllys ine66
N -Trimethyl-
3-OH-lys ine
Carnitine
Glutaryl-CoA S accharopine2-Aminoadipate
s emialdehyde
2-Aminoadipate2-Oxoadipate
Arginino-
s uccinate
Guanidoacetate
Methylmalonyl-CoA
4.1.2.12
As partyl
S emialdehyde
2, 3-Dihydro-
dipicolinate
1.2.1.11
4.2.1.52 1.3.1.26
Piperideine-
2, 6-dicarboxylate
N-S uccinyl-
2-amino-6-oxo-
pimelate
2.6.1.17
N-S uccinyl-2, 6
diaminopimelate
Diamino-
pimelate
3.5.1.18
3-Methyl-
glutaconyl-CoA
(CH ) CHCH COS CoA3 2 2(CH ) CHCH COS CoA3 2 2
3-Methyl-
crotonyl-CoA
Is ovaleryl-CoA
OxopantoateHCHO
OOCCH C = CHCOS CoA2
CH3
CH COCHCOS CoA3
CH3
CH CH=CHCOS CoA3
CH3
CH CH(OH)CHCOS CoA3
CH3
C(OH)CH(OH)COO
CH CH3 2
CH3
CH = CCOS CoA2
CH3
HOCH CHCOS -CoA-2
CH3
CH CHCO-S CoA3
CH3
C (OH)CH(OH)COO
CH3
CH3
CH CH CHCOS CoA3 2
CH3
CH C = CHCOS CoA3
CH3
ATP
4-Aminobutyrate
(GAB A)
1.3.99.7
1.5.1.2
1.14.11.2
2.6.1.39 1.2.1.31
1.14.11.8
2.5.1.16
2.5.1.22
4.1.3.16
2.6.1.23
1.5.99.8
1.5.1.2
6.4.1.4 1.3.99.10
4.2.1.33
2.6.1.6
1.1.1.85
Oxoleucine
COOH
(CH ) CHCHCH(OH)COO3 2
S -Adenos ylmethyl
thiopropylamine
(Decarboxylated S AM)
PR OLINE
Coenzyme A
CH C= CHCH3 2
CH3
O
CH
CH
3
3
HO
CH3CH3
CH3
CH C-CH H O3 2 2C PP
CH2
CH C= CHCH O2 2 PP
CH3
3-P-Glycerol
Phos phatidyl
ethanolamine
CE PHALIN
3.1.4.12
2.7.8.3
Proges terone
1.5.1.12
2.7.3.2
4.2.1.173.1.2.4
2.6.1.32
4.4.1.15
Acetyls erine 2.7.7.42.7.1.251.8.99.1
Adenylyls ulphate
(APS )
2.7.1.39
4.4.1.15
4.1.1.29
4.2.99.8
2.3.1.30
P-R ibos yl-PP
1.1.1.1
1.2.1.4
As partyl-P
5.1.1.7
2.7.1.40
4.1.3.18
4.1.3.18
1.5.1.9
1.14.11.1
CHOLINE
2.1.1.13
1.3.1.2
NHCH CH S H2 2NHCH CH CO2 2OCH C(CH ) CH(OH)CO2 3 2P
ADP- NHCH CH S H2 2NHCH CH CO2 2OCH C(CH ) CH(OH)CO2 3 2
P -ADP- NHCH CH S H2 2NHCH CH CO2 2OCH C(CH ) CH(OH)CO2 3 2
1.4.1.8
1.3.99.3
1.3.99.3
1.4.1.9
4.2.1.18
4.1.1.20
1.3.99.7
1.8.99.2
+
CH CO-OCH CH(NH )COO2 2 3
2.5.1.6
2.1.1.10
2.1.1.20
1.1.1.3
1.1.1.3
4.2.1.18
4.1.2.5
4.3.1.5
4.2.1.20
2.1.2.1
2.1.1.20
Glyoxylate
2.1.1.6
1.4.3.4
1.14.18.1
1.14.16.2
Hexanoyl-CoA
B utanoyl-CoA
6.3.4.16
6.3.5.5
2.7.2.11
2.1.3.3
NO
1.14.13.39
3.5.3.63.5.3.1
4.3.2.1
4.1.1.17
2.7.7.41
2.6.1.4
1.1.1.100
1.1.1.8
2.3.1.51
2.3.1.15
2.7.8.5
S O
-
4
2
1.14.16.4
NH2OOC
OOC
4.1.1.25
1.3.1.13
1.3.1.13
2.7.4.6
CYTIDINE -
triphos phate
(CTP)
Cytos ine
2.4.2.9
4.1.1.11
2.6.1.18
4.3.1.3
4.2.1.49
3HS O-
1.1.1.105
2.3.1.41
NADPH
1.1.1.22
2.3.1.4
5.5.1.4
3.2.1.26 3.2.1.48
2.6.1.16
1.1.1.14
2.7.7.34
4.2.1.47
2.4.1.68
2.4.1.69
2.4.1.9
5.3.1.8
2.4.1.11
O
OPPU
CH OH2
CH OH2
HO OH
OH
HO
2.4.1.21
4.2.1.52
1.2.1.18
4.2.1.18
O
CH
C
CHOC
N
HN
DP
CH
C
O
CHOC
N
HN
R PPP
OCH2
H
C C
H
OHOH
CH
NHN
C
H
CP
H
O
OCH2 C C
H
OH
H
OH
C
H
C C
C
NH2
C
CH
HC
N
R P(PP)
N
N
N
+
P
NH2H
O
OCH2 C C
H
OH
H
OH
C
H
C C
OC
C
CH
HC
N
R P
N
N
NHP
H
OCH2 C C
H
OHOH
CO CH2
C
CONH2
C
CH
HC
N
R P
N
N
NHP
+
OOCCH N(CH )2 3 3
B etaine
B etaine
aldehyde
2.4.1.16
5.1.3.6
2.4.1.33
2.7.7.13
5.4.2.8
5.3.1.8
O
OH
CH OH2
HO
O PHO
2.7.1.28
2.7.1.31
6.3.4.13
5.3.1.1
2.2.1.1
4.1.1.9
3.1.2.11
ß-OH-ß-Methyl-
glutaryl-CoA
6.3.4.5
5.3.1.6
2.5.1.21
2.1.1.2
3.5.2.10
6.3.2.13
6.3.2.7-10
2.7.7.27
2.7.7.9
3.5.4.10
C
C
O
C
CH
OC
N R P
N
N
H
HN
C
C
O
C
CH
N
N
N
HN
R P
H N2 C
2.7.4.6
1.17.4.1
2.7.4.6
2.6.1.13
Carbamoyl-P
2.3.1.9
Carnitine
2.6.1.4 2.6.1.44
CH C(OH)CH COS CoA3 2
CH COO2
1.2.3.5
1.1.1.34
2.3.1.16
4.1.3.5
2.3.1.16
4.1.3.4
Menaquinone
4.1.1.15
2.6.1.19
4.2.1.16
Carnos ine
CH CH NH2 2 2C
NHN
C
HC
H
4.1.1.22
4.3.1.3
Ubiquinone
Plas toquinone
D-R ibos e-5-P
NH
CH CH(NH )COO2 3HO
+
O
OH
OH
HO
OPPU
COO-
O
OH
OH
HO OPPU
COO -
OH
H OH
HOCH2 C C CO
H
CO COO -
O
CHOH
CHOHAcNH
HO
OPC
OCO -
CH OH2
O
CHOH
CHOHAcNH
HO
OH
COO
CH OH2
O
OHOH HOHO
CH O2 P
O
OH
ACNH
HO OH
CH O2 P
O
OOH HOHO P
CH OH2
O
OHOH HOHO
CH OH2
O
OHHO OH
3
CH O2 P
O
OHHO O
NHCOCH3
P
CH OH2CH OH2CH OH2
O
OHHO OH
NH2
CH O2 P
O
OH
HO
OH
OPPU
CH OH2
O
O
HO
OH
OH
HO
P
CH OH2
CH OH2
O
O
HO
OH
OH
O
OHOH
OH
CH OH2
O
OHHO
OH
OH
CH2CH OP2
OH
H H HOH
C C C C
OH OHH
COO-HOCH2
H
OH OHOH
C C C CHO
HH
HOCH2
H
OH OHH
C C C CHO
HOH
HOCH2
OH
H OHH
C C C CHO
HOH
HOCH2
H
OH HOH
C C C CHO
OHH
HOCH2
OH
H HOH
C C C CHO
OHH
HOCH2
OH
H OHOH
C C C CHO
HH
HOCH2
H
OH HOH
C C C C
OHH H
OH
COO-P OCH2
OH
H OH
C C CO
H
P OCH2 CH OH2
H
OH H
C C CO
OH
HOCH2
CH OH2
CH OH2P OCH2
H
OH OH
C C CO
H
CH OH2P OCH2
OH
H H
C C CO
OH
CH OH2
P OCH2
H
OH OH
C C CO
H
HOCH2
CH OH2
OH
H OH
C C CO
H
HOCH2
CH OH2
OH
H H
C C CO
OH
HOCH2 CH OH2
H
OH OHH
C C C
HOH
HOCH2 CH OH2
OH
H OHOH
C C C
HH
HOCH2
CH OH2
H
OH HOH
C C C C
OHH H
OH
CH OH2HOCH2
H
OH OH
C C CHO
H
POCH2
H
OH HOH
C C C CO
OHH
HOCH2
CH O2 P
H
OH OHOH
C C C
HH
HOCH2 CH OH2
H
OH OH
C C C
H
CO
H
HO
P OCH2
CH OH2
P
H
OH OH
C C C
H
CO
H
HO
CH O2 POCH2
H
OH OH
C C
H
C CHO
OH
H
POCH2POCH2
O
OH
NH2
OH
CH O2 P
CHOHCHOPOCH2
HOCH2COCH2OP
O
OH
O OP P
OH
CH O2 P
CHOHCOO PPOCH2
CHOHP OCH2 COO
COO
OH
OHO
OH
COO
CH (CH ) CH(OH)CH COS -CoA3 2 14 2CH (CH ) CH(OH)CH COS3 2 14 2
CH (CH ) COCH COS -CoA3 2 14 2
CH (CH ) COCH COS -CoA3 2 14 2
CH (CH ) COS -CoA3 2 n+2
CH (CH ) CH=CHCOS -CoA3 2 14
CH (CH )3 2 nCH=CHCOS -CoA
CH (CH )3 2 5 2CH=CHCH COS ACP
CH (CH )3 2 6CH=CHCOS ACP
CH (CH )3 2 6COCH COS ACP2CH (CH )3 2 6 2 2CH CH COS ACP
CH (CH )3 2 2 2 2CH CH COS ACP
CH CH CH COS ACP3 2 2
CH (CH )3 2 6CH(OH)CH COS ACP2
CH CH=CHCO.S -ACP3
CH (CH )3 2 2 2COCH COS ACP
CH3COCH COS ACP2
CH (CH )3 2 n 2COCH COS ACPCH (CH )3 2 n 2CH(OH)CH COS ACP
CH (CH )3 2 2 2CH(OH)CH COS ACP
AC YL-C oA
(Mitochondria)
CH (CH ) CH CH COS CoA3 2 2 2n
CH (CH ) CH=CHCOS CoA3 2 n CH (CH CH(OH)CH COS CoA3 2n
CH (CH ) CH CH COS CoA3 2 2 2 2 CH (CH ) CH=CHCOS CoA3 2 2
CH (CH ) COCH COS CoA3 2 2n
CH COCH COS CoA3 2
CH (CH ) CH(OH)CH COS CoA3 2 2 2
CH CH(OH)CH COS CoA3 2CH CH=CHCOS CoA3CH CH CH COS CoA3 2 2
CH CH CH CH COS CoA3 2 2 2
Pentenoyl-CoA
CH CH CH=CHCOS CoA3 2 CH CH CH(OH)CH COS CoA3 2 2
CH (CH ) COCH COS CoA3 2 2 2
CH CH COCH COS CoA3 2 2
OH OH
HO OH
O
O-
CH O-PO2
CH CHOHCH OH2 2
O
CH2
CH2
R '-CO-OCH
O-CO-R
O-PO
O-
HOCH CH2 2
NH3
+POCH CH2 2NH3
+
CPP- OCH CH2 2NH3
+
O
-
CH O-CO-R2
CH O P2 OCH CH2 2NH3
+
CH COCH CH N(CH )3 2 2 3 3
HOCH CH N(CH )2 2 3 3
+
CH CH2 COO(NH )3
+
OH
OH
CH CH2 COO-(NH )3
+
CO
NH2
CH CH(NH )COO2 3
+
NH
OC
CO
NH
OC
N
H
C
H
H N2
HC
CH
N
N
C
R P
H N2
CH
C
CHOC
N
N
DP
NH2
R PPP
CH
C
CHOC
N
N
NH2
C
C
C
CH
R P(P)
HC
N
N
N
N
NH2
H NCOO2 P
(CH ) NH2 3 2H N(CH ) NH2 2 3 (CH ) NH2 4
(CH ) NH2 3 2H N(CH ) NH2 2 4
CH -S CH CH CHNH3 2 2 2
Adenos yl
+
+
CH3
CH3
CHCH(NH )COO3
++
C
NHN
C
HC
H
CH CH(NH )CH OH2 3 2
+
C
NHN
C
HC
H
CH CH(NH )CH OP2 3 2
C
NHN
C
H
HC CH COCH OP2 2
+
HS CH CH CH(NH )COO2 2 3
+
+
S CH CH CH(NH )COO2 2 3
CH CH(NH )COO2 3
R P
C
O
C-COOOC
N
HN CH
2.4.1.23
CH (CH ) CH=CHCH(OH)CHCH OH3 2 12 2
NH3
+
CH (CH ) CH(OH)CHCH OH3 2 14 2
NH3
+
CH (CH ) COCHCH OH3 2 14 2
NH3
+
Galactos eCH (CH ) CH=CHCH(OH)CHCH O-3 2 12 2
NH3
+
4.1.1.70
H NCONH2 2
OHCCH N(CH )2 3 3
+
C-CH3
C
O
CHOC
N
DP
HN
DP
CPP-O CH CH N(CH )2 2 3 3
+
OCH CH N(CH )2 2 3 3
+
1.2.1.41
UDP-N-Ac-Muramate
O
O
CH CH3
COO-
NHAC
HO OPPU
CH OH2
4.1.3.20
O
OH
CH3
HO HO OPPG
2.7.7.24
2.4.1.22
2.7.7.12
5.1.3.2
5.3.1.4
S edoheptulos e-PP
3.1.1.28
4.2.1.24
N
CH2
CH2
N
N
N
CH3
CH3
CH
CH
COO
CH
CH
CH2
H C3
H C3H C3
HC
C
C
H
H
Fe
CH2
CH2
COO- -
CH3
CH3
CH2
CH2
CH2
H C3
H C3
N
H
N
H
N
H
N
H
CH
CH
H C2
C
C
H2
H2
-
CH2
CH2
COO
CH2
CH2
COO -
H C2
H C2
CH 2
CH 2
OOC
COO
H N2
N
H
-
-
CH2
H C2
H C2
H C2
H2
H2
CH2
CH2 CH2
CH2
CH2
CH2
N
H
N
H
N
H
N
H
COO
COO
COO
COO
C
C
-
-
CH2
CH2
COO
CH2
CH2
COO- -
-
-
OOC-
OOC
-
CH3
CH3
H C3
H C3
H C2
H2
H2
N
H
N
H
N
H
N
H
CH2
CH2
CH2
CH2
CH2
C
C
COO-
CH2
CH2
COO
CH2
CH2
COO- -
COO-
COO-
CH C(OH)CH CH O3 2 2 PP
CH COO2
OHCCOO
HOCH COO2
NH2
H NCNHCH COO2 2
+ NH2
H NCN(CH )CH COO2 3 2
+ NH2
HNCN(CH )CH COO3 2P-
+ HN C
NH CO
N CH2
CH3
OOCCHCH COO2
H NCNHCH CH CH CH2 2 2 2
N
(NH )3
+
COO
CHCOOHC
CH2HOCH
N
OOCCH(OH)CH CH(NH )COO2 3
+
CHCOO
CH2HOCH
H C2
N
H
NH
CHCOO
CH2CH2
CH2
OOCCH(OH)CH COCOO2
N
CHCOO
CH2CH2
CH
CH COCOO3 OHCCOO
+
H NCONHCH CH CH CH2 2 2 2 (NH )3
COO
H NOCCH CH CH2 2 2 COO(NH )3
+
R -CO-COO
+
COOR -CH(NH )3
+
COOH NOCCH CH2 2 (NH3
+
OOC-CH-COS CoA
CH3
P OOCCH CH2 COO(NH )3
+
OOCCH CH CHO2 2
OHCCH CH2 COO(NH )3
+
(CH ) NCH CH(OH)CH COO3 3 2 2
+
OHCCH CH CH2 2
+
COO(NH )3
POOCCH CH CH2 2
+
COO(NH )3
OHCCH CH CH CH2 2 2 COO(NH )3
+
NH CHCH CH COO2 2
COO
CH CH CH CH CH2 2 2 2 COO(NH )3
+
H N(CH )2 42 CH(NH )COO3CH(NH3
++
(CH ) N(CH )3 3 2 3CH CH(NH )COO2 3
+ +
C H2
C
H
OOCC
N
HC
CH-COO
CH2
C
H2
CH-COOOOCC
N
H C2
OOCCOCH CH CH CH-COO2 2 2CH CH2 2 2
OOCCH CH CONH2 2 OOCCHCH CH CH CH-COO2 2 2
OOCCH CH CONH2 2CH2 2
NH3
+
OOCCH-CH CH CH CHCOO2 2 2
NH3
+
NH3
+
(CH ) CHC(OH)CH3 2 2
COOH
COO (CH ) CHCH COCOO3 2 2
+
(CH ) CHCH3 2 2
CH(NH )COO3
HOCH CHCOO2
CH3
CHCOCOO
CH3
CH3CH COC(OH)CH3 3
COO
+
P OCH CH CH(NH )COO2 2 3
+
HOCH CH CH(NH )COO2 2 3
+
CH CH CH(NH )COO2 2 3OOCCH CH2COO2
+
CH S H2
OOCCH(NH )CH CH CONHCHCONHCH COO3 2 2 2
HOCH C(CH ) COCOO2 3 2
HOCH C(CH ) CH(OH)COO2 3 2
NHCH CH COO2 2HOCH C(CH ) CH(OH)CO3 2)2 3 2
NHCH CH COO2 2OCH C(CH ) CH(OH)CO2 3 2P
CH CH3 2
CH3
CH
+
CH(NH )COO3
CH COC(OH)CH CH3 2 3
COO CHCOCOO
CH CH3 2
CH3
NHCHCH S H2
COO
NHCH CH CO2 2OCH C(CH ) CH(OH)CO2 3 2P
+
CH S H2
OOCCH(NH )CH CH CONHCHCOO3 2 2
+
Adenosyl
S CH CH CH(NH )COO2 2 3
+
S CH CH CH(NH )COO2 2 3
+
HO S CH CH(NH )COO3 2 3
+
+
S -CH CH(NH )COO2 3
S -CH CH(NH )COO2 3
+
HS CH CH(NH )COO2 3
1.1.1.23
H
C
NHN
C
HC CH CHCOO2
NHCOCH CH NH2 2 2
+
H
C
NHN
C
HC CH CH(NH )COO2 3
CHCH CH COO2 2
NHN
CH
OC C CH CHCOO
NHN
CH
CH
CH COO3
N
H
OOC
CH-COOOC
NH2 CH2
H NCH CH COO2 2 2
H NCH2 2
CH3
CHCOO H NCONHCH CHCOO2 2
CH3
C
C
O
CH
R P
HC
N
N
N
HN
C
CC
O
CH
HCO
N
N
N R P
C
CH
N
N
H N2
H
C
C
C
O
CH
N
N
C
C
CH
N
N
R P
H N2
H N2
C
O
O
O P~ ~P O
O
O O
P O
O
CH2
N
N
N
N
O
OHOH
NH2
HC
CH
N
N
N
N
O
O
P O O
O
CH2
OH
NH2
HC
CH
COO
CO
NH
OC
N
H
C
H
H N2
NH2
OOC-CH-CH COO2
HNCO C
CH
N
N
C
R PR P
H N2
OOC
C
CH
N
R P
N
C
H N2
CH=CHCOO
OH
CH=CHCOO
CH COCOO2
CH2
P
COO
OH
O
O
O
COO
O
CH COO2
O
CH COO2-OOC
OCH2 CH2
OHH
C
P
HOCH HCOH
OC
COO
COOHO
OH
OHO
NH2
COO
OCH
NH
CH COCOO2
NH
CHO
CO
NH2
OH
CH CH(NH )COO2 3
+
N
COO
COO
N
+
COO
COO
R P
O
OH
OH
HO OPPU
COO
O
OH
NHCOCH3
HO
OPPU
COO
CH OH2
O
O
CCH2 NHAC
HO OPPU
COO
CH OH2
O
OHHO OH
OH
COO
OOCCH N(CH )2 3 2
Dimethylglycine
1.1.99.1
1.2.1.8
COO
HO HO
COO
O
HO OH
COO
CH(O )PP OCH2 COO
CH(OH)HOCH2 COOCOO
CH(O )PHOCH2 COO
CH =C(O )2 P COO
CONH2
R ibose -O - P - O - P - O- Adenosine(P)
+
N
O
O
O
O
+
COO
N
R ibose - O - P - O - P - O -Adenosine
O
O
O
O
II
II
OH
O
CH CH2 COO(NH )3
+
E pinephrine
(Adrenaline)
Norepinephrine
(Noradrenaline)
Normetepinephrine
(Normetadrenaline)
HOCH CH N (CH )2 2 3 3
+
Adenosyl
+
+
S CH CH CH(NH )COO2 2 3
CH3
(CH ) N(CH )3 3 2 3CH CH(NH )COO2 3
+ +
OH
OOCCH CH CH COS CoA2 2 2
OOCCH CH CH NH2 2 2 2
OOCCH CH CH COCOO2 2 2 OOCCH CH CH CH2 2 2 COOCOO(NH )3
++
HO
H
HO
H
H
HO
H
HO
H
Pros taglandin PGE2
OH
HOCH2 C C CO C COO
OHH
OHH H
-
COO
OR '-CO-OCH
CH O-CO-R2
CH O POCMP2
O
CH CH N(CH )2 2 3
+
OHOCH
CH OPO2
CH OH2
O-
CH OPO2
O
CH OCH=CHR2
CH CH N(CH )2 2 3 3
+
O-
CH OPO2
OR '-CO-OCH
CH O-CO-R2
3.1.1.32
CH CH N(CH )2 2 3 3
+
O-
OHOCH
CH OPO2
CH O-CO-R2
CH CH N(CH )2 2 3 3
+
O-
CH CH(NH )COO2 3
+
+
C
NHN
C
HC
H
CH CH(NH )CHO2 3
HS
11-cis -R etinal
Pi
ADP
NADP +
GDP-
Mannuronate
Mannos e-6-P
Dehydro-
s hikimate
S hikimate
R NA
d-GDP
d-CTP
GUANOS INE -P
(GMP)
ß-Alanine
P-R ibos yl-AMP P-R ibulos ylformimino
5-aminoimidazole-
carboxamide-R P
Imidazole
acetol-P
HS HS O-
PANTOTHE NATE
3-Is opropyl-
malate
S uccinic
s emialdehyde
2.4.1.17
Diacyl
glycerol
2.3.1.6
1.14.12.1
1.4.4.2
CH CHO3
3.5.4.19
4.2.1.19
3.3.1.1
4.1.1.12
4.1.2.5
COO-
2OHCCH
2.1.4.1
2.4.99.7
5.1.3.12
1.1.1.132
4.2.1.46
2.7.1.7
2.4.1.29
2.4.1.21
2.4.1.13
2.4.1.1
etc.
5.4.2.2
2.7.1.6
2.7.7.10
3.1.1.18
4.1.1.34
2.7.1.47
2.7.1.16
3.1.3.9
ADP
P-Glucono
lactone
1.1.1.44
O
O
OHHO
OH
CH O2 P
PHE NYLALANINETYR OS INE
TR YPTOPHAN
INOS INE -P
(IMP)
THYMIDINE -P
HIS TIDINE
UR IDINE -
triphos phate
(UTP)
CYS TINE CYS TE INE
IS OLE UCINE
LE UCINE
LYS INE
OR NITHINE
AR GININE
HYDR OXY
PR OLINE
P HOS P HATIDYL
S E R INEPhos phatidyl
inos itol
LE CITHIN
S PHINGOMYE LIN
S TE R OIDS
Pregnenolone
2.7.6.1
2.4.2.14
6.3.4.7
5.3.1.9
3.1.3.11
1.2.1.13
2.7.2.3
H
OH OH
C C C
H
CO
OH
C
H
H
OH
P OCH2
CH O2 P
5.4.2.1
ADP
4.2.1.11
E ndoplas mic R eticulum
1.1.1.100
3.1.1.3
Phos phatidate
2.3.1.39
4.2.1.17
4.2.1.17
4.2.1.55
1.1.1.79
1.2.1.21
1.4.3.8
1.1.1.32
2.7.1.36
2.7.4.2
4.1.1.33
2.5.1.1
2.5.1.10
2.7.8.5
2.7.8.1
4.1.1.65
2.7.7.14
1.3.99.7
PO
O
CH (CH ) CH=CHCH(OH)CHCH O3 2 12 2
NHAcyl
CH CH N(CH )2 2 3 3
+
O-
3.5.1.23
1.13.11.21
2.5.1.32
1.1.1.105
2.4.2.19
1.2.1.32
ME THIONINE
4.1.3.27
4.2.1.51
1.14.17.1
1.14.18.1
1.14.13.11
Glycinamide-
ribos yl-P
4.2.1.22
3-Oxohexanoyl-CoA
THR E ONINE
1.2.1.25
1.2.1.25
1.2.1.25
1.5.1.7 - 10
GLUTAMATE
2.3.1.38
ATP
NADH
S UCR OS E
ADE NOS INE -P
(AMP)
Triacylglycerol
FAT
R NA
CH3
CH2
OC-COOOH
COO
CH CH2 COO(NH )3
+
GLYCOPR OTE INS
GANGLIOS IDE S
MUCINS
4.1.2.-
2.6.1.-
GLUCOS E
O-Acyl-carnitine
O-Acyl-carnitine
OH
O-
-
R -CO-OCH
R '-CO-OCH
R '-CO-OCH
O HCO-CO-R
CH CH(OH)CH O-P-OCH2 2 2
CH O-CO-R ’2
OR '-CO-OCH
O -
CH O-CO-R2
CH O-PO2
O-
2.7.8.11
H NCH CH CH CH2 2 2 2 COO(NH )3
+
2.3.1.20 3.1.3.4 2.7.1.107
NH2
+
+
H NCNHCH CH CH CH2 2 2 2 COO(NH )3
H
OH HOH
C C C CO
OHH
HOCH2
CH OH2
R -CH2COO
CH O-CO-R2
CH O-CO-R "2
R ’-CO-OCH
OH
O
CH CHNH2 3
+
OR '-CO-OCH
O-
CH O-CO-R2
CH O PO2
COO
OOCCH CH CH2 2
COO(NH )3
+
CH CH(OH)CH(NH )COO3 3
O
OHHO
OH
OH
CH OH2CH OH2
NH
CH O-CO-R2
1.3.1.13
4.1.1.28
Malonic
s emi-
aldehyde
2.7.2.4
2.1.3.3
3.5.1.2
6.3.1.2
6.3.5.5
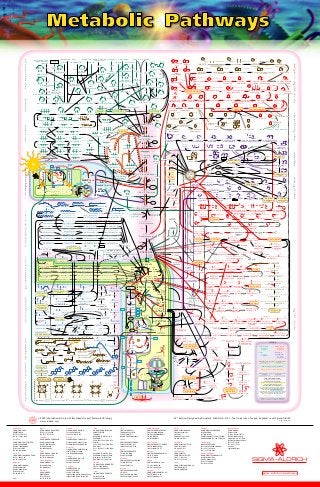
Carbohydrates
Biosynthesis
Degradation
Amino Acids
Biosynthesis
Degradation
Lipids
Biosynthesis
Degradation
Purines &
Pyrimidines
Biosynthesis
Degradation
Biosynthesis Degradation
Vitamins Co-enzymes & Hormones
Pentos e Phos phate Pathway
Photos ynthes is Dark R eactions
COMPAR TME NTATION
The "Backbone" of metabolism involves
GLYCOLYS IS in the CYTOPLAS M,
the TCA CYCLE (mainly) in the Mitochondrial matrix
and ATP FOR MATION spanning the
MITOCHONDR IAL INNE R ME MBR ANE
An electron flow (an electric current) generated from
NADH and UQH2 drives the translocation of protons
from the matrix to the intermembrane space.
The retrolocation of these protons through the F0 subunits
of ATP synthase to the matrix then supplies the energy
needed to form ATP from ADP and phosphate
E lectron Flow Proton Flow
S mall Numbers ( eg. 2.4.6.7) refer to the IUBMBE nzyme
Commission (E C) R eference Numbers of E nzymes
LE GE ND
Human Metabolism is identified as far possible by black arrows
Biosynthesis Degradation
HE MOGLOB IN CHLOR OPHYLL
ATP
TRANSLOC
A
T E D PR OTONS
2.7.1.1
ATP
2.7.1.2
sigma-aldrich.com/pathways
Argentina
SIGMA-ALDRICH DE
ARGENTINA, S.A.
Tel: 54 11 4556 1472
Fax: 54 11 4552 1698
Australia
SIGMA-ALDRICH PTY., LIMITED
Free Tel: 1800 800 097
Free Fax: 1800 800 096
Tel: 612 9841 0555
Fax: 612 9841 0500
Austria
SIGMA-ALDRICH HANDELS GmbH
Tel: 43 1 605 81 10
Fax: 43 1 605 81 20
Belgium
SIGMA-ALDRICH NV/SA.
Free Tel: 0800-14747
Free Fax: 0800-14745
Tel: 03 899 13 01
Fax: 03 899 13 11
Brazil
SIGMA-ALDRICH BRASIL LTDA.
Tel: 55 11 3732-3100
Fax: 55 11 3733-5151
Canada
SIGMA-ALDRICH CANADA LTD.
Free Tel: 800-565-1400
Free Fax: 800-265-3858
Tel: 905-829-9500
Fax: 905-829-9292
China
SIGMA-ALDRICH CHINA INC.
Tel: 86-21-6386 2766
Fax: 86-21-6386 3966
Czech Republic
SIGMA-ALDRICH s.r.o.
Tel: 246 003 200
Fax: 246 003 291
Denmark
SIGMA-ALDRICH
DENMARK A/S
Tel: 43 56 59 10
Fax: 43 56 59 05
Finland
SIGMA-ALDRICH FINLAND
Tel: 358-9-350-92 50
Fax: 358-9-350-92 555
France
SIGMA-ALDRICH CHIMIE S.à.r.l.
Tel appel gratuit: 0800 211 408
Fax appel gratuit: 0800 031 052
Germany
SIGMA-ALDRICH CHEMIE GmbH
Free Tel: 0800-51 55 000
Free Fax: 0800-649 00 00
Greece
SIGMA-ALDRICH (O.M.) LTD
Tel: 30 210 9948010
Fax: 30 210 9943831
Hungary
SIGMA-ALDRICH Kft
Tel: 06-1-235-9054
Fax: 06-1-269-6470
Ingyenes zöld telefon: 06-80-355-355
Ingyenes zöld fax: 06-80-344-344
India
SIGMA-ALDRICH CHEMICALS
PRIVATE LIMITED
Telephone
Bangalore: 91-80-5112-7272
Hyderabad:
91-40-5531 5548 / 2784 2378
Mumbai:
91-22-2579 7588 / 2570 2364
New Delhi:
91-11-2616 5477 / 2619 5360
Fax
Bangalore: 91-80-5112-7473
Hyderabad: 91-40-5531 5466
Mumbai: 91-22-2579 7589
New Delhi: 91-11-2616 5611
Ireland
SIGMA-ALDRICH IRELAND LTD.
Free Tel: 1800 200 888
Free Fax: 1800 600 222
Israel
SIGMA-ALDRICH ISRAEL LTD.
Tel: 08-948-4100
Fax: 08-948-4200
Italy
SIGMA-ALDRICH S.r.l.
Telefono: 02 33417310
Fax: 02 38010737
NumeroVerde: 800-827018
Japan
SIGMA-ALDRICH JAPAN K.K.
TokyoTel: 03 5821 3111
Tokyo Fax: 03 5821 3170
Korea
SIGMA-ALDRICH KOREA
Tel: 031-329-9000
Fax: 031-329-9090
Malaysia
SIGMA-ALDRICH (M) SDN. BHD
Tel: 603-56353321
Fax: 603-56354116
Mexico
SIGMA-ALDRICH QUÍMICA,
S.A. de C.V.
Free Tel: 01-800-007-5300
Free Fax: 01-800-712-9920
The Netherlands
SIGMA-ALDRICH CHEMIE BV
Tel Gratis: 0800-0229088
Fax Gratis: 0800-0229089
Tel: 078-6205411
Fax: 078-6205421
New Zealand
SIGMA-ALDRICH PTY., LIMITED
Free Tel: 0800 936 666
Free Fax: 0800 937 777
Norw ay
SIGMA-ALDRICH NORWAY AS
Tel: 23 17 60 60
Fax: 23 17 60 50
Poland
SIGMA-ALDRICH Sp. z o.o.
Tel: (+61) 829 01 00
Fax: (+61) 829 01 20
Portugal
SIGMA-ALDRICH QUÍMICA, S.A.
Free Tel: 800 20 21 80
Free Fax: 800 20 21 78
Russia
SIGMA-ALDRICH RUSSIA
TechCare Systems, Inc.
(SAF-LAB)
Tel: 095-975-1917/3321
Fax: 095-975-4792
Singapore
SIGMA-ALDRICH PTE. LTD.
Tel: 65-6271 1089
Fax: 65-6271 1571
South Africa
SIGMA-ALDRICH
SOUTHAFRICA (PTY) LTD.
Tel: 27 11 979 1188
Fax: 27 11 979 1119
Spain
SIGMA-ALDRICH QUÍMICA S.A.
Free Tel: 900101376
Free Fax: 900102028
Sw eden
SIGMA-ALDRICH SWEDEN AB
Tel: 020-350510
Fax: 020-352522
Outside SwedenTel: +46 8 7424200
Outside Sweden Fax: +46 8 7424243
Sw itzerland
FLUKA CHEMIE GmbH
Swiss Free Call: 0800 80 00 80
Tel: +41 81 755 2828
Fax: +41 81 755 2815
United Kingdom
SIGMA-ALDRICH COMPANY LTD.
Free Tel: 0800 717181
Free Fax: 0800 378785
Tel: 01747 833000
Fax: 01747 833313
United States
SIGMA-ALDRICH
P.O. Box 14508
St. Louis, Missouri 63178
Toll-free: 800-325-3010
Call Collect: 314-771-5750
Toll-Free Fax: 800-325-5052
Tel: 314-771-5765
Fax: 314-771-5757
Internet:
sigma-aldrich.com
© 2003 International Union of Biochemistry and Molecular Biology
www.iubmb.org
22nd
Edition Designed by Donald E. Nicholson, D.Sc., The University of Leeds, England – and Sigma-Aldrich
Product No. M 3907
XXX