This document provides an introduction and preface to the proceedings of the special session on shrimp biotechnology held at the 5th Asian Fisheries Forum in Chiengmai, Thailand in November 1998. It was edited by T.W. Flegel of the Department of Biotechnology at Mahidol University and the National Center for Genetic Engineering and Biotechnology (BIOTEC) in Thailand. The proceedings contain over 40 papers on various topics related to shrimp biotechnology, including maturation and genetics, shrimp rearing, the shrimp defense system, probiotics and immunostimulants, bacterial diseases and toxins, and viral diseases.








































































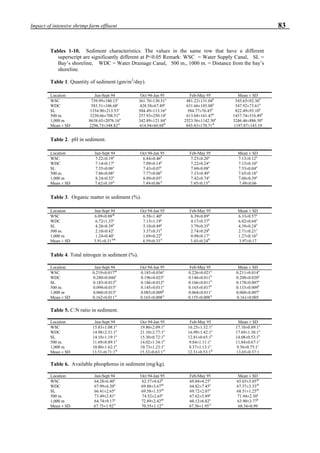








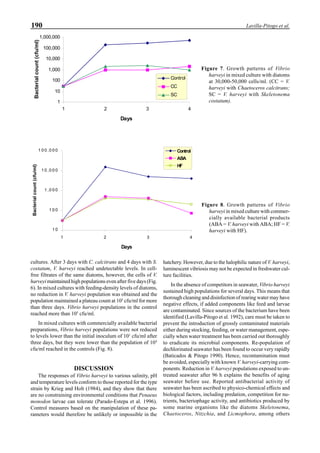
![95Ozonation of shrimp ponds
IN SITU APPLICATION OF OZONE IN
SHRIMP PONDS
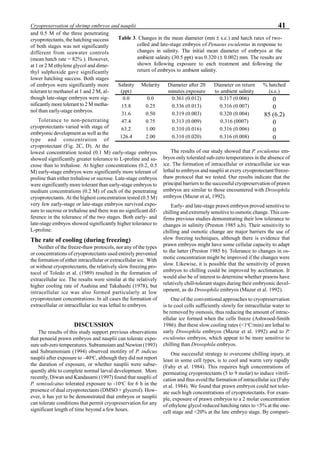
Water-jet circulator
There is a consensus among aquaculturists that water cir-
culation in ponds is beneficial. Water circulation prevents
thermal and chemical stratification. This makes the entire
pond volume habitable and it eliminates oxygen depletion at
the sediment-water interface. During daylight hours, surface
water in ponds is often supersaturated with DO produced by
phytoplankton, and water at greater depths may have low
DO concentration. The paddle wheel aerator is commonly
employed in intensive culture ponds, because it blends sur-
face water with subsurface water. By mixing pond water, a
uniform DO profile can be established, and the total DO
content of a pond can be increased.
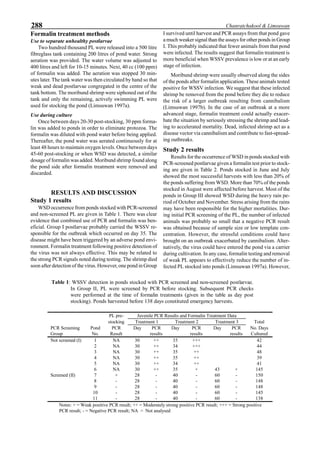
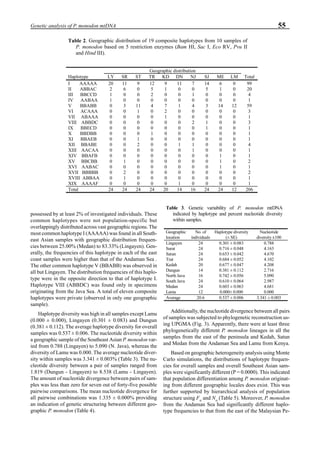
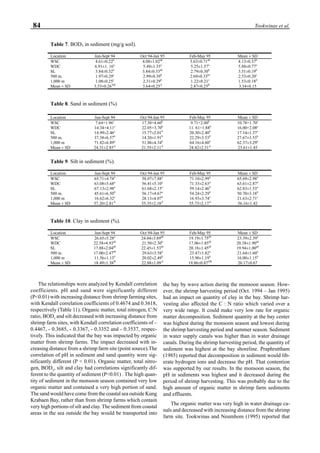
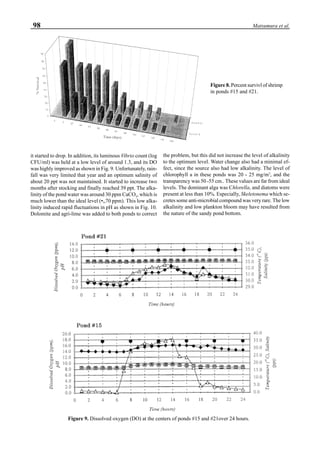
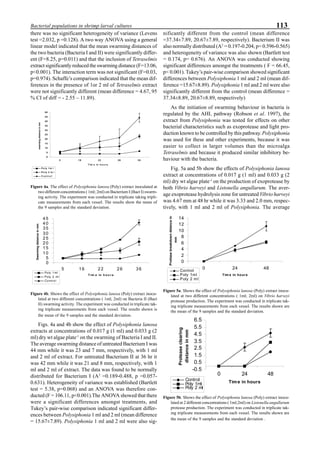
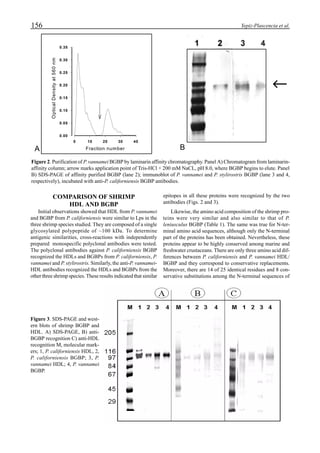
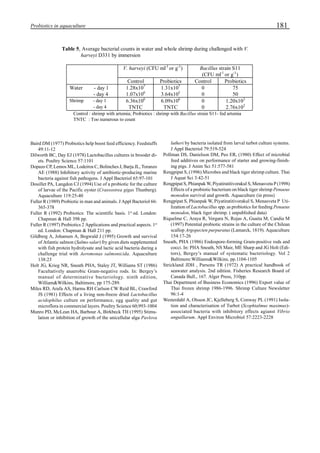
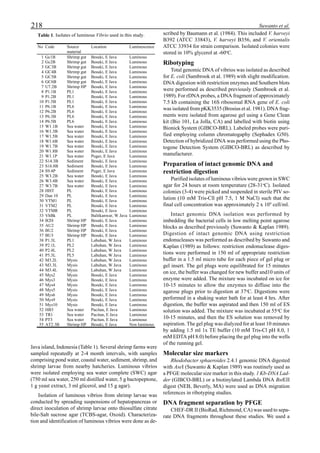
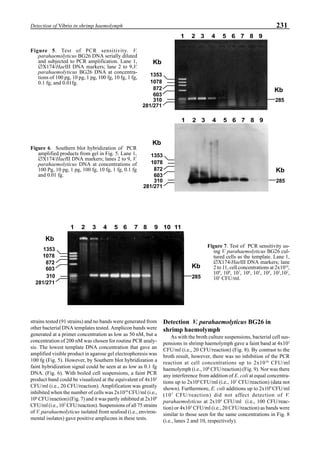
Figure 3 shows the 24-hour monitoring of DO at two
different sampling points (SP-1 and SP-2) in an intensive
culture pond with a surface area of 4500 m2
and 1.5 m depth.
Four paddle wheel aerators were installed in the pond. One
had 6 blades and the others had 3 blades. They were driven
by 1 horsepower motors. The monitoring was conducted on
the 150th
day after stocking. The locations of sampling points
SP-1 and SP-2 were the mid point between two paddle wheel
aerators and the center of pond, respectively. At the sam-
pling point SP-1, there was a liquid mixing enough to dimin-
ish the vertical distribution of DO between the pond surface
and the bottom. However, an obvious difference in DO be-
tween the surface and the bottom could be seen at sampling
point SP-2. This was caused by poor liquid mixing. It was
clear that uniform water quality in the pond could not be
attained by the paddle wheel aerators. Therefore, we intro-
duced a water-jet circulator to enhance liquid mixing.
The construction of the water-jet circulator was based
on a double stage ejector as shown in Fig. 4. It was installed
at the bottom of the pond as shown in the system construc-
tion. We could also introduce ozone mixed with air into the
nacelle of the jet pump and make very fine bubbles which
were easily dispersed in the sea water. When the driving liq-
uid was supplied into the jet pump through a narrow slit at a
volumetric flow rate of Q1
, the liquid was sucked at a flow
rate of Q2
. The driving force for the liquid suction is pro-
vided by the pressure drop of the driving liquid. Then, the
liquid is discharged from the nacelle at a flow rate of Q1
+Q2
. This discharged liquid acts as the driving liquid for the
second ejector. The final discharged flow rate becomes
Q1
+Q2
+Q3
. The design of the ejector has been well estab-
lished, and the energy efficiency of the jet pump ā is ex-
pressed by the following equation:
η = [Q1
/Q2
][(hd
- h2
)/(h1
- hd
)] ≡ M·H (11)
where, h1
, h2
, hd
are the hydrostatic head at the pump con-
nection points for drive line, suction line and discharge line,
Figure 3. Dissolved oxygen (DO) in a shrimp pond
aerated with paddle wheels (∆=Top; ¨=Middle;
•••••=Bottom).
Figure 4. Schematic for construction of the water jet circulator.](https://image.slidesharecdn.com/advancesinshrimpbiotechnology-181105043109/85/Advances-in-shrimp-biotechnology-95-320.jpg)
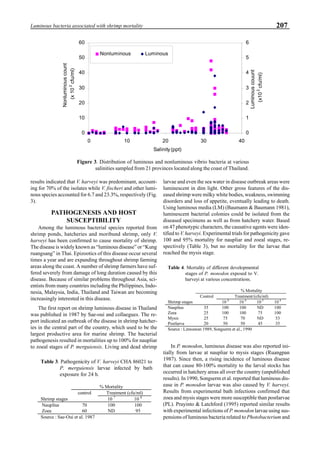
![96 Matsumura et al.
respectively. For a jet pump with a low discharge pressure
but a high suction flow rate, a low area ratio of about 0.2 is
recommended for the construction of the nozzle. In this case,
M becomes 2.2, so the discharged liquid volume becomes
3.2 times as much as that of the drive liquid. For the double
jet pump, this becomes 10.2 times.
Once the jet flow is established, the liquid surrounding
the jet stream is entrained into the stream. The volumetric
flow rate of entrained liquid QE
is given by Eq.(12):
QE
= [0.203(x/do
-1)]Qd
(12)
where Qd
is the volumetric flow rate discharged from the
water-jet circulator (Q1
+Q2
+Q3
), and do
is the diameter of
open duct shown in Fig. 4. X is the maximum distance from
the exit edge of the open duct where the jet flow exits. If x/do
is 40, QE
in Eq.(12) becomes 8.2Qd
, and then QE
/Q1
is 83.5.
When a water-jet circulator with a driving liquid volume of
0.75 m3
/min is installed in a shrimp pond with a liquid vol-
ume of 6,000 m3
, the pond liquid passes through the circula-
tor at a volumetric flow rate of 11,000 m3
/d. Therefore, the
circulation time for the pond water becomes 0.54 d. A rather
huge amount of liquid is entrained into the jet stream at a
volumetric flow rate of 90,000 m3
/d, and this liquid flow
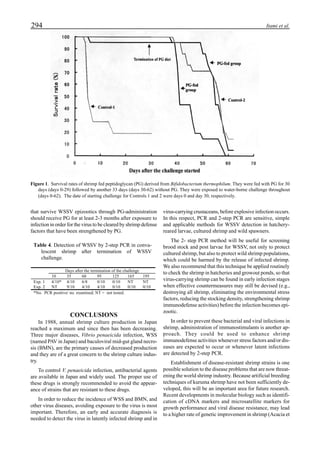
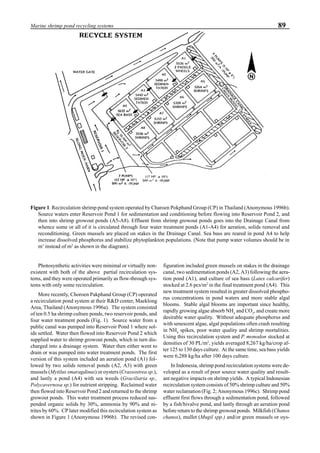
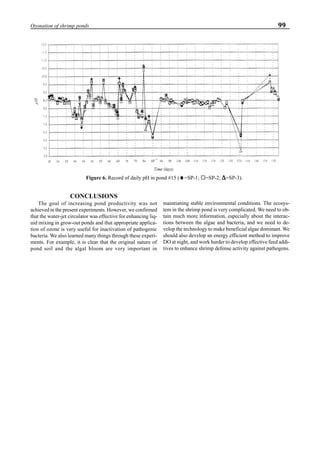
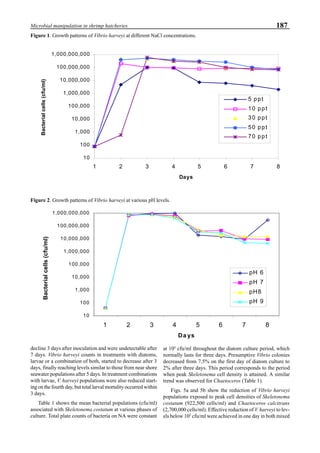
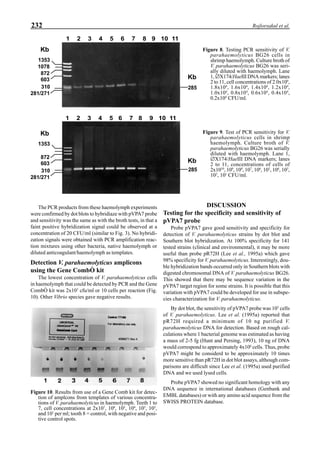
will enhance the mixing of pond liquid. Figure 5 shows the
24- hour monitoring of DO in the same pond shown in Fig.
3. In this case, one water-jet circulator (driving liquid vol-
ume: 0.75 m3
/min, energy consumption: 2.2 kW) was in-
stalled together with two paddle wheel aerators. As you can
see, the liquid mixing was greatly improved, and the vertical
distribution of DO was diminished even at the center of the
pond.
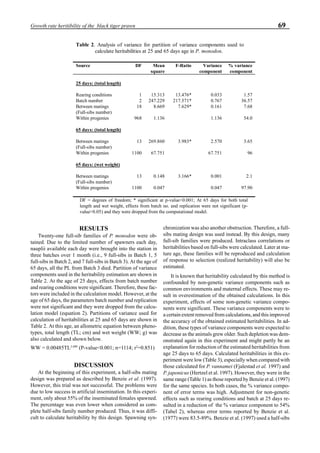
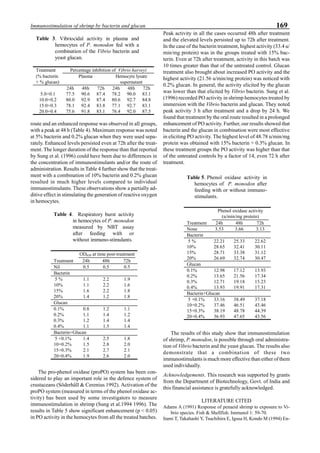
Effects of ozone on shrimp growth
The first experiment of shrimp cultivation using ozone
was conducted in a shrimp farm located at Calatagan in the
Luzon Island (Philippines) where no problems caused by
pathogenic bacteria and viruses occurred. The main purpose
of this experiment was to investigate whether ozone had any
adverse effects on shrimp growth. An ultraviolet ozone gen-
erator containing two 110 w lamps was used under an air
flow rate of 100 l/min. The exit ozone concentration was
100 ppm. The ozone dosing rate into the culture pond was
0.22 mg-O3
/h/m3
.
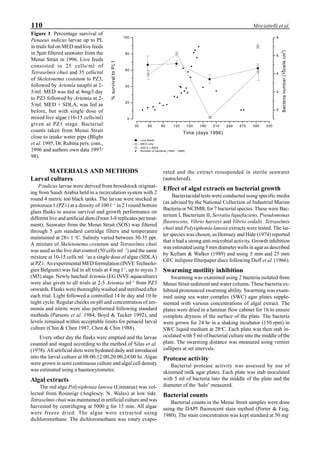
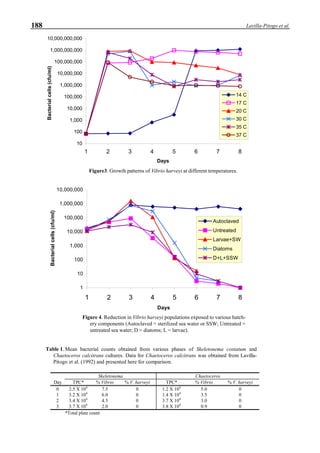
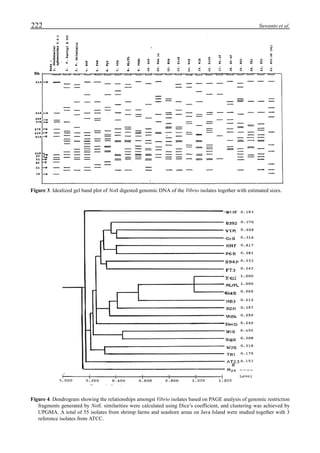
Figure 6 shows the average body weight over time for
shrimp cultivated in three different ponds, pond #3 (surface
area; 4000 m2
), #9 (4500 m2
) and #15 (4000 m2
). One water-
jet circulator and two paddle wheels were installed in ponds
#3 and #9 using sea water with an average salinity of 32 ppt,
but ozone was employed only in pond #3. Pond #15 was
operated with four conventional paddle wheels, and shrimp
Figure 5. Vertical dissolved oxygen (DO) distribution in a pond using a water-jet circulator (♦♦♦♦♦=SP-1 top;¨=SP-1 bottom,
∆=SP-2 top; X=SP-2 bottom; ∗∗∗∗∗= SP-3 top; •••••=SP-3 bottom).
Figure 6. Average body weight of shrimp in ponds #3, #9 and #15 (♦♦♦♦♦=pond #3; ¨=pond #9, ∆=pond #15).](https://image.slidesharecdn.com/advancesinshrimpbiotechnology-181105043109/85/Advances-in-shrimp-biotechnology-96-320.jpg)


















![120 Darachai et al.
DISCUSSION
Haematococcus cultivation
H. pluvialis grew well under laboratory conditions at
25o
C. With high light intensity stress, it accumulated
astaxanthin and turned red. Astaxanthin esters are the pri-
mary pigment in H. pluvialis cysts, typically ranging from
60% to 80% by weight of the total pigment content (Spencer
1989). Kobayashi et al. (1992) showed that H. pluvialis
NIES144 accumulated astaxanthin mainly in esterified form
[monoester (69%) and diester (20%)]. By contrast, free
astaxanthin is the major compound in synthetic astaxanthin.
The predominant configuration isomer in H. pluvialis is 3S,
3’S (Johnson & An 1991). This 3S, 3’S configuration is also
found in lobster (Homarus gammarus) eggs and yeast
(Phaffia rhodozyma).
Effect of dietary astaxanthin on shrimp
larvae
From our results, the highest survival rate of zoea and
mysis was obtained with shrimp fed AAD followed by NF,
NAD and SAD, in descending order. This indicated that
shrimp larvae accept natural better than synthetic astaxanthin.
Commonly, the early stages of shrimp larvae are fed with
phytoplankton (e.g., Chaetoceros sp.) and zooplankton (e.g.,
Artemia sp.) from which they derive natural carotenoids (and
convert them to astaxanthin). In the case of AAD, it con-
tained H. pluvialis that had produced natural astaxanthin and
accumulated various nutrients (from the rich cultivation me-
dium) and these could be utilized directly by the larvae. By
contrast, the free form of astaxanthin in SAD may have been
difficult for early larval stages to utilize and this may have
affected their survival. Indeed, the zoea fed SAD had the
lowest survival.
In postlarval stages, there were no significant differences
in survival rate for larval groups fed AAD, SAD and NAD.
The postlarvae fed NF had the lowest survival (significantly
lower than the postlarvae on the AAD diet). This indicated
that Artemia nauplii as the sole feed to postlarvae could not
provide sufficient nutrients for good survival. Chindamaikul
and Phimonchinda (1990) reported that the survival of P.
monodon from zoea to postlarva-15 was higher when they
were fed natural feed (Chaetoceros sp. and Artemia sp.) plus
artificial feed than it was when they were fed only natural
feed or only artificial feed.
Efficacy of astaxanthin was higher for postlarvae fed
AAD than for those fed NF (Artrmia sp.). Tanaka et al. (1975)
explained that Artemiasp. accumulated mainly canthaxanthin
and that two biochemical steps were required for shrimp to
convert it to astaxanthin. By contrast, the astaxanthin in H.
pluvialis cysts could be used directly. The source of
astaxanthin may affect survival rate of shrimp larvae differ-
ently at different stages. For example, the survival of early
larval stages fed the diet containing synthetic astaxanthin
(SAD) was poor but survival was higher for larvae at the
postlarval stage. Utilization of free astaxanthin may be poor
for zoea and mysis but may be better for postlarvae. Both
zoeal and mysis stages naturally consume algae and small
living organisms (e.g., rotifers and Artemia) which are natu-
ral sources of astaxanthin.
The postlarva-15 fed natural diets containing natural
astaxanthin were larger than those fed diets containing syn-
thetic astaxanthin or no astaxanthin. The best postlarval
growth was in the group fed AAD and this was significantly
better than that for groups fed SAD. This indicated that
astaxanthin from H. pluvialis (mostly in esterified form) per-
formed significantly better than free, synthetic astaxanthin.
Brown et al. (1991) reported that shrimp fed a dry diet plus
algae grew faster and had better survival than shrimp fed a
dry diet only. This was probably because the algae contained
essential nutrients that were lacking in the dry diet. Simi-
larly, H. pluvialis NIES144 was cultured in a rich medium
containing thiamin as a growth factor and its dry cysts con-
tained approximately 40% protein and 1% fat. These factors
may explain why H. pluvialis cysts in the diet were more
advantageous than synthetic pigments. Cohen (1986) re-
ported that dietary xanthophylls from algae are incorporated
into the exoskeleton of prawns and lobsters (as astaxanthin)
where they play a major role in pigmentation. Other func-
tions are poorly defined. Boonyaratpalin et al. (1994) found
that addition of synthetic astaxanthin and canthaxanthin pig-
ment to diets did not effect growth and feed efficiency of P.
monodon juveniles.
The pigment content of shrimp fed pigment-free diets
was less than that of groups fed pigmented diets. This con-
firmed that shrimp larvae can accumulate carotenoids (mainly
astaxanthin) from their diets. The astaxanthin content of
shrimp fed NF (Artemia sp.) was higher than that of other
groups. It may because Artemia sp. contains various
carotenoids, such as astaxanthin, violaxanthin, zeaxanthin,
echinenone, b-carotene, lutein in addition to its main pig-
ment canthaxanthin. Some of these carotenoids can be con-
verted to astaxanthin. Moreover, all carotenoids have simi-
lar maximum absorption wavelengths that may overlap or
interfere during analysis by spectrophotometer. The larvae
fed AAD contained higher amounts of carotenoids. This may
have resulted for several reasons. For example, shrimp lar-
vae may gain esterified astaxanthin and accumulate it in body
tissue better than free astaxanthin. Secondly, the larvae may
be more accustomed to natural astaxanthin than synthetic
astaxanthin. Third, they may have accept AAD better than
SAD. However, the factors controlling pigment absorption,
transportation and excretion among various shrimp tissues
are not known. Herring (1969) speculated that pigment ab-
sorbed in excess an animal’s requirement is later excreted.
Determination of 50% cumulative mortality upon low
salinity challenge showed that larvae fed AAD endured bet-
ter than larvae fed NF, SAD and NAD. In addition, toler-
ance of shrimp fed pigmented diets (AAD and SAD) was
higher than those fed a pigment-free diet (NAD). Astaxanthin
seemed to be helpful to the postlarvae and to prolong their
life upon this acute environmental stress.
The esterified astaxanthin in AAD was a storage form of
the pigment and it was probably accumulated in the lipid
portion of the larval hepatopancreas before transfer to other
sites in the body by haemolymph (Ghidalia 1985). This was
probably also true for the free astaxanthin in SAD. How-
ever, P. monodon has been reported to accumulate esterified
astaxanthin (85.7%) more than free astaxanthin (14.3%)
(Latscha 1989).](https://image.slidesharecdn.com/advancesinshrimpbiotechnology-181105043109/85/Advances-in-shrimp-biotechnology-120-320.jpg)














































































































![Loh PC, Cesar E, Nadala BJr., Tapay LM, Lu Y (1998) Recent developments in immunologically-
based and cell culture protocols for the specific detection of shrimp viral pathogens. In Flegel TW
(ed) Advances in shrimp biotechnology. National Center for Genetic Engineering and Biotechnol-
ogy, Bangkok.
Recent Developments in Immunologically-Based and
Cell Culture Protocols for the Specific Detection of
Shrimp Viral Pathogens
1
Philip C. Loh, 1
E. Cesar, B. Nadala Jr., 3
Lourdes M. Tapay, 2
Yuanan Lu
1
Department of Microbiology, 2
Retrovirology Laboratory, University of Hawaii, Honolulu, Hawaii, 96822, U.S.A.
3
National Institute of Molecular Biology and Biotechnology, University of the Philippines
Los Baños, Philippines
ABSTRACT: A Western blot protocol capable of detecting YHV and CBV (WSSV) in the hemolymph of
infected shrimps was developed. This protocol was highly specific and rapid, and sensitive enough to detect the
presence of the viruses before the appearance of overt symptoms. It was also useful in demonstrating the growth
of both viruses in primary lymphoid cell cultures. A flow chart for routine shrimp virus pathogen detection and
identification employing the more convenient but less specific NC-EIA as a presumptive test followed by the
highly reliable WB protocol as a confirmatory assay was advanced. The combined protocols, together with the
primary shrimp cell culture system, have been successfully used in field surveillance of shrimp hatcheries and
farms.
KEY WORDS: Shrimp virus; Pathogen; Detection; Western blot; Enzyme immunoassay; cell culture.
INTRODUCTION
Shrimp viral diseases have seriously impacted the
sustainability and economic success of the shrimp aquaculture
industry worldwide. Among several of the recent viral patho-
gens which have caused massive mortalities in cultured
shrimp are included yellow-head virus (YHV) and white spot
syndrome virus (WSSV) (also called Chinese baculovirus
[CBV] or systemic ectodermal and mesodermal baculovirus
[SEMBV]). Both YHV and CBV (WSSV) have been re-
ported by our laboratory to be highly pathogenic for Penaeus
stylirostris (blue shrimp) and P. vannamei (white shrimp),
the two principal penaeid species commercially cultured in
Hawaii and the Western Hemisphere (Lu et al. 1997). They
are thus potentially serious problems particularly to the
broodstock industry.
A major problem for the control and prevention of shrimp
viral diseases is the lack of relatively simple and cost-effec-
tive technologies for the early detection and diagnosis of
viral infections, particularly asymptomatic infections. Con-
trol and eradication of the virus problem would be much
more expeditious with an efficient detection/diagnostic test
to identify infected animals long before they showed clini-
cal signs. This is particularly true for asymptomatic
broodstock carriers. In this paper, we report the successful
development of a less invasive, combined Western blot and
enzyme immunoassay protocol for the early detection of both
YHV and CBV (WSSV) in experimentally infected animals,
before the appearance of clinical signs of disease. Primary
shrimp cell cultures were also used for the detection of both
viruses. These protocols were successfully employed for the
detection and isolation of both CBVand YHV from field sam-
ples.
MATERIALS AND METHODS
Preparation of anti-YHV and anti-CBV
antibodies
Polyclonal antibody against YHV and CBVwere prepared
in New Zealand white rabbits (7-8 lbs) using purified virus
as antigen. Immunoglobulin G was purified from the antis-
era using recombinant bacterial protein-G columns (Gamma-
Bindä). In order to remove antibodies that cross-reacted with
normal shrimp antigens, the IgG was adsorbed onto acetone-
dried, ground shrimp muscle tissue as well as shrimp
hemolymph.
SDS-polyacrylamide gel electrophoresis
(SDS-PAGE)
SDS-PAGE was done according to the method of
Laemmli (1970). Briefly, samples were suspended in load-
ing buffer (0.5 mM Tris-Cl, pH 6.8, 2.5 ml; 10% sodium
dodecyl sulfate, 4 ml; glycerol, 2 ml; ß-mercaptoethanol, 1
ml; and deionized distilled water, 0.5 ml), boiled at 95°C for
5 min, loaded into the wells of 5% (YHV) and 10% (CBV)
SDS-PAGE, and electrophoresed at 200 V.
Western blotting
The electrophoresed gel was blotted onto a nitrocellu-
lose membrane (pore size, 0.1 mm) in blotting buffer (3.03g](https://image.slidesharecdn.com/advancesinshrimpbiotechnology-181105043109/85/Advances-in-shrimp-biotechnology-255-320.jpg)
![256 Loh et al.
Tris base, 14.4g glycine, & 200ml methanol per liter) at 100
V for 1 hr. The membrane was then rinsed in PBS (pH 7.4),
soaked in 5% skim milk (in PBS) for 1 hr, and rinsed in PBS
for 5 min. The membrane was treated with 1:1000 dilution
of the primary antibody (either a polyclonal hyperimmune
anti-YHV IgG or anti-CBV IgG) for 1 hr, rinsed 3X with
PBS for 5 min, and then treated with 1:2500 dilution of the
secondary antibody (goat anti-rabbit IgG-horseradish per-
oxidase conjugate [Kirkegaard and Perry Laboratories, Inc.,
Gaithersburg, MD]) for 1 hr. The membrane was again rinsed
3X with PBS for 5 min and then treated with the substrate
(3,3’
,5,5’
-tetramethylbenzidine or TMB [Kirkegaard and
Perry Laboratories, Inc., Gaithersburg, MD]) until a bluish
purple color developed. The reaction was stopped by soak-
ing the membrane in distilled water. All of the incubations
were done at room temperature (r.t., 25°C ±2°C) unless speci-
fied otherwise.
Time-course infectivity experiments in whole
animals
Ten Penaeus vannamei (40-60 gram) were injected
intramuscularly in the second segment of the abdomen with
either 5% w/v of head soft tissues from CBV -infected shrimp
or 10% w/v head soft tissues from YHV-infected shrimp at
0.2 ml per shrimp. At specific periods after injection, 0.1 ml
hemolymph was collected from the hemocoel of at least 2
randomly selected shrimps using a 1 ml syringe with 26G
needle. The hemolymph were diluted in equal volume of 20%
citrate buffer and then stored at -80°C until needed.
For Western blotting, 200 µl aliquots of hemolymph sam-
ples were clarified at 8,000 x g for 5 min and then pelleted at
140,000 x g for 5 min. The pellets were resuspended in 100
ml 2x loading buffer and heated to 95°C for 5 min. Only 10
µl of the treated samples were loaded per well of the SDS-
PAGE gel.
Time-course infectivity experiments in pri-
mary shrimp lymphoid cell cultures
Forty-eight hr primary shrimp lymphoid cell cultures in
25 cm2
PrimariaTM
flasks were inoculated with 0.5 ml of 100-
fold dilution of CBV or YHV filtrate (clarified and filtered
10% w/v virus-infected head soft tissues). Adsorption was
carried out at r.t. for 1.5 hr after which the excess filtrate was
removed and replaced with 5 ml of growth medium (2X
Leibovitz Medium-15 supplemented with 8% shrimp head
extract, 5% fetal bovine serum, 6 ml salt solution, 100 I.U./
100 mg/ml penicillin/streptomycin). The flasks were incu-
bated at r.t. and were sampled at various days post-infection
(p.i.) by freezing at -80°C. An uninoculated flask was fro-
zen at 7 days and used as control. The flasks were then thawed
and collected into each of sterile conical tubes for centrifu-
gation at 1800 x g for 15 min at 5°C. The supernatant was
stored at -80°C until needed.
For western blotting, the samples were clarified by cen-
trifuging 200 µl aliquots at 8,000 x g for 5 min and then
pelleting them at 140,000 x g for 5 min. The pellets were
resuspended in 20 µl 2x loading buffer and heated to 95°C
for 5 min. All 20 µl of the treated samples were loaded in the
well of the SDS-PAGE gel.
RESULTS
Detection of viral proteins by NC-EIA
The nitrocellulose-enzyme immunoassay (NC-EIA) pro-
tocol when used for the detection of viral proteins was found
to have a sensitivity of 0.4 ng for YHV and 1 ng for CBV.
The polyclonal antisera used also exhibited strong cross-re-
actions with hemolymph and normal shrimp tissues. Such
cross-reactivities however, could be significantly reduced by
prior extensive adsorption with hemolymph. Since residual
cross-reactivity could not be completely eliminated, the
adsorbed antisera for both YHV and CBV were then em-
ployed in the Western blot format where virus-specific pro-
teins were clearly dilineated from normal hemolymph and
tissue proteins.
Detection of YHV proteins in the hemolymph
and in primary cell cultures
Protein analysis of purified virus preparations by SDS-
PAGE revealed four major bands with the following esti-
mated molecular sizes (kDa): 175, 135, 67 and 22 (Tapay et
al. 1997). These bands probably corresponded to the large
(L), glycoprotein (G), nucleocapsid (N) and matrix (M) pro-
teins of rhabdoviruses. Western blot analysis of these bands
using polyclonal anti-YHV IgG showed strong reactivity of
the antibody with the putative G protein band, as well as the
L and N bands.
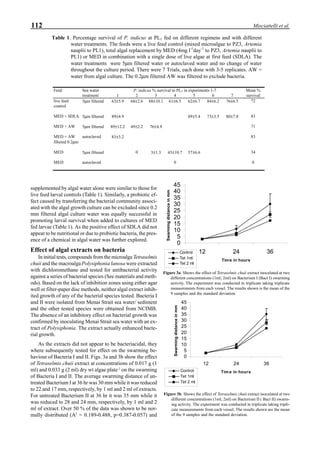
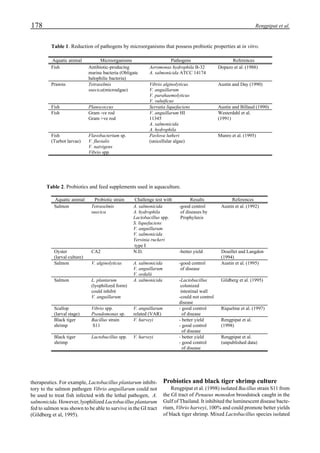
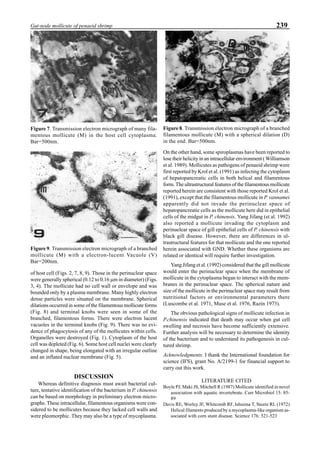
Western blot analysis of YHV-containing hemolymph
samples obtained from experimentally-infected shrimp
showed that, despite some cross-reaction with normal
hemolymph proteins, the putative G protein (135kDa) could
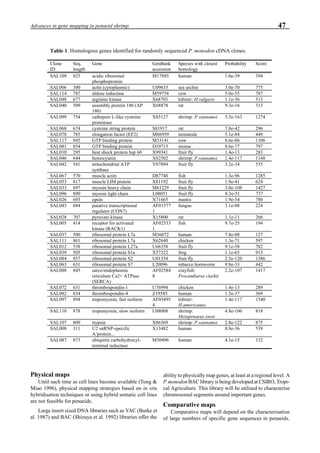
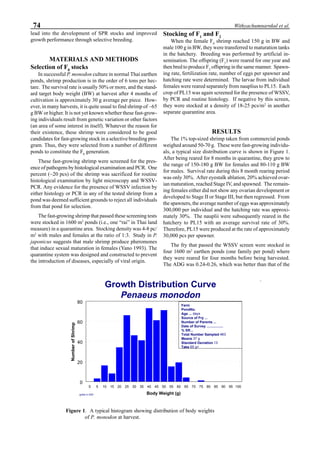
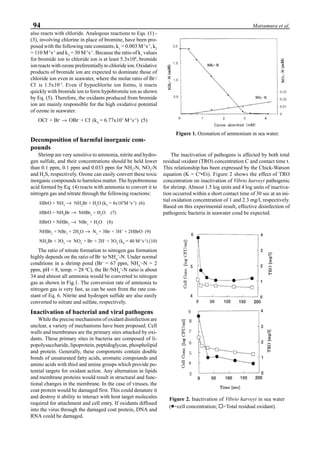
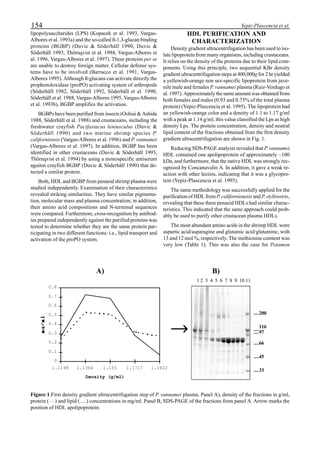
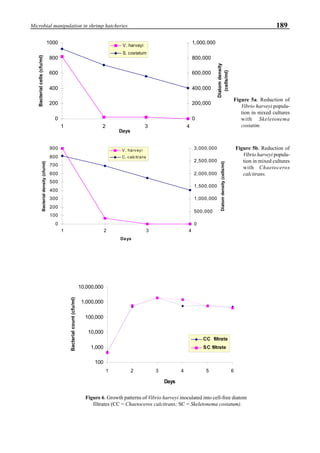
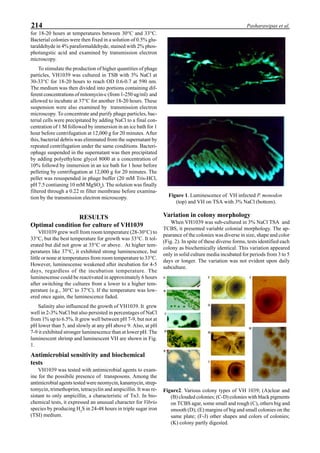
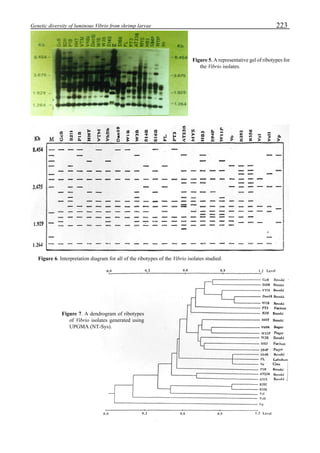
be specifically distinguished. Figure 1 shows that the viral
protein first became detectable in the hemolymph 56 hours
p.i.. Overt symptoms in infected animals were not observed
until 72 hours p.i.
Western blot analysis of YHV-infected primary lymphoid
cell cultures had similar results. The putative G protein of
the virus was easily distinguished from background stain-
ing, and was detected as early as 4 days p.i. (Fig. 2).
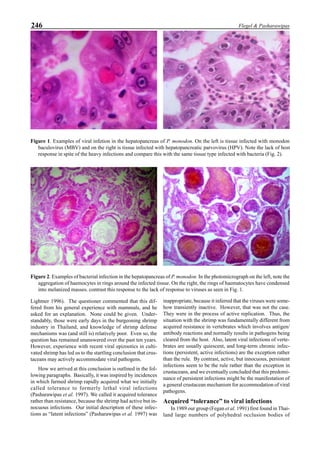
Figure 1. Western blot of YHV proteins (135 & 170 kDa,
arrowheads) in hemolymph samples collected at various pe-
riods from shrimp (P. vannamei) experimentally infected with
YHV.](https://image.slidesharecdn.com/advancesinshrimpbiotechnology-181105043109/85/Advances-in-shrimp-biotechnology-256-320.jpg)





















![Lo CF, Chang YS, Cheng CT, Kou GH (1998) PCR monitoring of cultured shrimp for white spot
syndrome virus (WSSV) infection in growout ponds. In Flegel TW (ed) Advances in shrimp biotech-
nology. National Center for Genetic Engineering and Biotechnology, Bangkok.
*e-mail: ghkou@ccms.ntu.edu.tw
PCR Monitoring of Cultured Shrimp for
White Spot Syndrome Virus (WSSV) Infection
in Growout Ponds
1
Chu-Fang Lo, 1
Yun-Shiang Chang, 2
Chin-Te Cheng, *1
Guang-Hsiung Kou
1
Department of Zoology, National Taiwan University, Taipei, Taiwan, ROC
2
Tainan City Animal Disease Diagnosis Control Center, Tainan, Taiwan, ROC
ABSTRACT: White spot syndrome (WSS) is a viral disease which affects most of the commercially cultivated
marine shrimp species, not just in Asia but globally. WSS can cause up to 100% mortality, with a correspond-
ingly devastating economic impact. So far no significant resistance to this disease has been reported for any
species of shrimp. The causative agent of WSS, white spot syndrome virus (WSSV) is extremely virulent and
has a wide host range. Based upon the sequence of the most conserved region of white spot syndrome virus
(WSSV), a nested WSSV polymerase chain reaction (PCR) has been developed into one of the most powerful
diagnostic tool available to date. It is very sensitive, being able to detect 20 copies of target DNA in a PCR
reaction. In the present study, in addition to the 2-step nested WSSV PCR that we reported previously, we also
used single-step nested WSSV PCR to monitor the cultured shrimp for WSSV infection during their growth
period. Based on the single-step nested WSSV PCR results, WSSV infection can be divided into very severe
infection (level 1), severe infection (level 2), light infection (level 3) and very light infection (level 4). In our
previous paper, we classified WSSV infection into three stages, that is the asymptomatic carrier, transition and
patent stages. In asymptomatic carriers, the infection was at levels 3-4, while that in transition and patent states,
the infection was at levels 1-2. While the carrier stage may persist for months, under certain triggering condi-
tions (e.g., stress), it may also progress to the transition and patent stages within a few hours to a few days. We
found that shrimp at infection levels 3 and 4 were able to survive from the larval stages through till they were
harvested. As expected, once the infection of the cultured shrimp became level 1-2, mortality inevitably oc-
curred within a few days. PCR can thus predict mortality, but unfortunately by the time this prediction can be
made (i.e., at the transition stage) mortality is unavoidable. The challenge at this point is to design a PCR test
which will warn of an impending transition stage in time to take [stress reducing] action to prevent mass mortal-
ity.
KEY WORDS: WSSV infection in shrimp, monitoring by PCR.
INTRODUCTION
White spot syndrome (WSS) is a viral disease which af-
fects most of the commercially cultivated marine shrimp spe-
cies, not just in Asia but globally (Chou et al. 1995, Lightner
1996, Flegel 1997, Lotz 1997, Span & Lester 1997). In 1993,
WSS was first observed in cultured Penaeid shrimp in Tai-
wan (Chou et al. 1995). Since then, WSS has become wide
spread and the most economically damaging disease of cul-
tured shrimp in Taiwan. White spots in the exoskeleton and
epidermis are the most commonly observed clinical sign of
WSS in diseased shrimp. However, the presence of white
spots does not always mean that the condition is terminal.
For instance, under non-stressful conditions, infected shrimp
that have white spots may survive indefinitely. However, if
the shrimp also appear: lethargic, if their color changes to
pink or reddish-brown, if they gather around the edges of
ponds at the surface during the day, or if there is a rapid
reduction in food consumption, then a very high mortality
rate in the shrimp population can be expected within a few
hours to a few days of the onset of the signs. WSS can cause
up to 100% mortality, with a correspondingly devastating
economic impact. Lightner (1996) pointed out that no sig-
nificant resistance to this disease had been reported for any
species of shrimp, and this still remains true today. The causa-
tive agent of WSS, white spot syndrome virus (WSSV) is
extremely virulent and has a wide host range (Lo et al. 1996b).
Based upon the sequence of the most conserved region of
this virus, a 2-step nested WSSV polymerase chain reaction
(PCR) has been developed into one of the most powerful
diagnostic tool available to date. With its high specificity
and sensitivity, WSSV diagnostic PCR can be used for
screening carriers in shrimp larvae, parental spawners and
invertebrate populations that share the same habitat as well
as in helping to ascertain the transmission and infection cy-
cle of WSSV. WSSV diagnostic PCR is also suitable for
monitoring cultured shrimp for WSSV infection during their
growth period (Lo et al. 1998).
Our laboratory is presently carrying out a field survey of
WSSV infection in cultured shrimp in more than 50 earthen](https://image.slidesharecdn.com/advancesinshrimpbiotechnology-181105043109/85/Advances-in-shrimp-biotechnology-281-320.jpg)




![286 Lo et al.
amplicons will result as the severity of the infection increases
(L. Liu and C. Su of Farming IntelliGene Tech. Co. pers.
comm.). Thus, infected shrimp can be graded in severity ac-
cording to their PCR product profiles.
PCR can predict mortality, but unfortunately by the time
this prediction can be made (i.e., at the transition stage)
mortality is unavoidable. The challenge at this point is to
design a PCR test which will warn of an impending transi-
tion stage in time to take [stress reducing] actions to prevent
mass mortality. On the other hand, since stressful conditions
frequently cause mass mortality not only in diseased shrimp
but also in healthy shrimp populations, an alternative – or
complementary – approach would be to identify a detect-
able common key response of shrimp to stressful conditions
so that a rapid detection method could be developed to iden-
tify shrimp that are under stress.
Acknowledgments. This work was supported by the Council
of Agriculture under grant no. 87-AST-1.4-FID-06 (12-3).
We are indebted to Mr. Leo Liu and Mr. Chen Su, Farming
IntelliGene Tech. Co. for numerous discussions concerning
the Detection Kit for WSSV used in the present study and
their constructive suggestions for PCR template preparation.
We are indebted to Mr. Paul Barlow for his helpful criticism
of the manuscript.
LITERATURE CITED
Chou HY, Huang CY, Wang CH, Chiang HC, Lo CF (1995) Patho-
genicity of a baculovirus infection causing white spot syndrome
in cultured penaeid shrimp in Taiwan. Dis Aquat Org 23:165-
173
Flegel TW (1997) Special topic review: Major viral diseases of the
black tiger prawn (Penaeus monodon) in Thailand. World jour-
nal of microbiology & Biotechnology 13: 433-442
Lightner DV (ed) (1996) A handbook of pathology and diagnostic
procedures for diseases of penaeid shrimp. World Aquaculture
Soc, Baton Rouge, LA, Section 3.11
Lo CF, Kou GH (1996) WSBV 1461 bp Sal I fragment. EMBL/
GenBank Data Libraries.
Lo CF, Leu JH, Ho CH, Chen CH, Peng SE, Chen YT, Chou CM,
Yeh PY, Huang CJ, Chou HY, Wang CH, Kou GH (1996 a)
Detection of baculovirus associated with white spot syndrome
(WSBV) in penaeid shrimps using polymerase chain reaction.
Dis Aquat Org 25: 133-141
Lo CF, Ho CH, Peng SE, Chen CH, , Hsu HC, Chiu YL, Chang
CF, Liu KF Su MS, Wang CH, Kou GH (1996 b) White spot
syndrome baculovirus (WSBV) detected in cultured and cap-
tured shrimp, crabs and other arthropods. Dis Aquat Org 27:
215-225
Lo CF, Ho CH, Chen CH, Liu KF, Chiu YL, Yeh PY, Peng SE,
Hsu HC, Liu HC, Chang CF, Su MS, Wang CH, Kou GH (1997)
Detection and tissue distribution of white spot syndrome
baculovirus (WSBV) in captured brooders of Penaeus monodon
with a special emphasis on reproductive organs. Dis Aquat Org
30: 53-72
Lo CF, Hsu HC , Ho CH, Tsai MF, Kou GH, Lightner DV (1998)
Specific genomic DNA fragment analysis of different geo-
graphical isolates of shrimp white spot syndrome associated
virus. Dis Aquat Org (in press)
Lo CF, Kou GH (1998) Virus-associated white spot syndrome of
shrimp in Taiwan: A review. Fish Pathol 30: (in press)
Lotz JM (1997) Special review: Viruses, biosecurity and specific
pathogen-free stocks in shrimp aquaculture. World journal of
microbiology & Biotechnology 13: 405-413
Spann KM, Lester RJG (1997) Special topic review: Viral dis-
eases of penaeid shrimp with particular reference to four vi-
ruses recently found in shrimp from Queensland. World jour-
nal of microbiology & Biotechnology 13: 419-426](https://image.slidesharecdn.com/advancesinshrimpbiotechnology-181105043109/85/Advances-in-shrimp-biotechnology-286-320.jpg)