eBook University Physics for the Life Sciences, 1e Randall Knight, Brian Jones, Stuart Field.pdf
- 2. Get Complete eBook Download Link below for instant download https://browsegrades.net/documents/2 86751/ebook-payment-link-for-instant- download-after-payment
- 3. This page is intentionally left blank
- 4. Useful Data Earth data and gravity Me Mass of the earth 5.98 * 1024 kg Re Radius of the earth 6.37 * 106 m g Free-fall acceleration 9.80 m/s2 G Gravitational constant 6.67 * 10-11 N # m2 /kg2 Thermodynamics kB Boltzmann constant 1.38 * 10-23 J/K R Gas constant 8.31 J/mol # K NA Avogadro’s number 6.02 * 1023 particles/mol T0 Absolute zero -273°C patm Standard atmosphere 101,000 Pa u Atomic mass unit (Dalton) 1.66 * 10-27 kg Speeds of sound and light vsound Speed of sound in air at 20°C 343 m/s c Speed of light in vacuum 3.00 * 108 m/s Electricity and magnetism K Coulomb constant (1/4pP0) 8.99 * 109 N # m2 /C2 P0 Permittivity constant 8.85 * 10-12 C2 /N # m2 m0 Permeability constant 1.26 * 10-6 T # m/A e Fundamental unit of charge 1.60 * 10-19 C Quantum and atomic physics h Planck constant 6.63 * 10-34 J # s 4.14 * 10-15 eV # s U Planck constant 1.05 * 10-34 J # s 6.58 * 10-16 eV # s aB Bohr radius 5.29 * 10-11 m Particle masses mp Mass of the proton (and the neutron) 1.67 * 10-27 kg me Mass of the electron 9.11 * 10-31 kg Common Prefixes Prefix Meaning femto- 10-15 pico- 10-12 nano- 10-9 micro- 10-6 milli- 10-3 centi- 10-2 kilo- 103 mega- 106 giga- 109 terra- 1012 Conversion Factors Length 1 in = 2.54 cm 1 mi = 1.609 km 1 m = 39.37 in 1 km = 0.621 mi Velocity 1 mph = 0.447 m/s 1 m/s = 2.24 mph = 3.28 ft/s Mass and energy 1 u = 1.66 * 10-27 kg 1 cal = 4.19 J 1 eV = 1.60 * 10-19 J Time 1 day = 86,400 s 1 year = 3.16 * 107 s Force 1 lb = 4.45 N Pressure 1 atm = 101 kPa = 760 mm Hg 1 atm = 14.7 lb/in2 Rotation 1 rad = 180°/p = 57.3° 1 rev = 360° = 2p rad 1 rev/s = 60 rpm Greek Letters Used in Physics Alpha a Nu n Beta b Pi Π p Gamma Γ g Rho r Delta ∆ d Sigma g s Epsilon P Tau t Eta h Phi Φ f Theta 𝚹 u Psi c Lambda l Omega Ω v Mu m Mathematical Approximations (1 + x)n ≈ 1 + nx if x V 1 sinu ≈ tanu ≈ u and cosu ≈ 1 if u V 1radian ln11 + x2 ≈ x if x V 1
- 5. Brief Contents PART I Force and Motion 2 Chapter 1 Physics for the Life Sciences 4 2 Describing Motion 25 3 Motion Along a Line 52 4 Force and Motion 90 5 Interacting Systems 124 6 Equilibrium and Elasticity 163 7 Circular and Rotational Motion 199 8 Momentum 230 9 Fluids 256 PART II Energy and Thermodynamics 308 Chapter 10 Work and Energy 310 11 Interactions and Potential Energy 337 12 Thermodynamics 378 13 Kinetic Theory 422 14 Entropy and Free Energy 459 PART III Oscillations and Waves 506 Chapter 15 Oscillations 508 16 Traveling Waves and Sound 543 17 Superposition and Standing Waves 578 PART IV Optics 620 Chapter 18 Wave Optics 622 19 Ray Optics 656 20 Optical Instruments 692 PART V Electricity and Magnetism 728 Chapter 21 Electric Forces and Fields 730 22 Electric Potential 770 23 Biological Applications of Electric Fields and Potentials 811 24 Current and Resistance 842 25 Circuits 871 26 Magnetic Fields and Forces 907 27 Electromagnetic Induction and Electromagnetic Waves 949 PART VI Modern Physics 990 Chapter 28 Quantum Physics 992 29 Atoms and Molecules 1025 30 Nuclear Physics 1058
- 6. This page is intentionally left blank
- 7. University Physics FOR THE LIFE SCIENCES
- 8. This page is intentionally left blank
- 9. F University Physics FOR THE LIFE SCIENCES RANDALL D. KNIGHT California Polytechnic State University, San Luis Obispo BRIAN JONES Colorado State University STUART FIELD Colorado State University With contributions by Catherine Crouch, Swarthmore College
- 10. Please contact https://support.pearson.com/getsupport/s/ with any queries on this content. Cover Image by SCIEPRO, Science Photo Library Copyright © 2022 by Pearson Education, Inc. or its affiliates, 221 River Street, Hoboken, NJ 07030. All Rights Reserved. Manufactured in the United States of America. This publication is protected by copyright, and permission should be obtained from the publisher prior to any prohibited reproduction, storage in a retrieval system, or transmission in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise. For information regarding permissions, request forms, and the appropriate contacts within the Pearson Education Global Rights and Permissions department, please visit www.pearsoned.com/permissions/. Acknowledgments of third-party content appear on the appropriate page within the text or on pages C-1 and C-2, which constitutes an extension of this copyright page. PEARSON, ALWAYS LEARNING, and MasteringTM Physics are exclusive trademarks owned by Pearson Education, Inc. or its affiliates in the U.S. and/or other countries. Unless otherwise indicated herein, any third-party trademarks, logos, or icons that may appear in this work are the property of their respective owners, and any references to third-party trademarks, logos, icons, or other trade dress are for demonstrative or descriptive purposes only. Such references are not intended to imply any sponsorship, endorsement, authorization, or promotion of Pearson’s products by the owners of such marks, or any relationship between the owner and Pearson Education, Inc., or its affiliates, authors, licensees, or distributors. Library of Congress Cataloging-in-Publication Data Names: Knight, Randall Dewey, author. | Jones, Brian, author. | Field, Stuart, author. Title: University physics for the life sciences / Randall D. Knight, California Polytechnic State University, San Luis Obispo, Brian Jones, Colorado State University, Stuart Field, Colorado State University; with contributions by Catherine Crouch, Swarthmore College. Description: Hoboken: Pearson, 2021. | Includes index. | Summary: “University Physics for the Life Sciences has been written in response to the growing call for an introductory physics course explicitly designed for the needs and interests of life science students anticipating a career in biology, medicine, or a health-related field”—Provided by publisher. Identifiers: LCCN 2020053801 | ISBN 9780135822180 (hardcover) | ISBN 9780135821053 (ebook) Subjects: LCSH: Physics—Textbooks. Classification: LCC QC23.2 .K658 2021 | DDC 530—dc23 LC record available at https://lccn.loc.gov/2020053801 Cataloging-in-Publication Data is on file at the Library of Congress. Access Code Card ISBN-10: 0-135-82129-0 ISBN-13: 978-0-135-82129-9 Rental ISBN-10: 0-135-82218-1 ISBN-13: 978-0-135-82218-0 ScoutAutomatedPrintCode Content Development Director HE Content Management Science & Health Sciences: Jeanne Zalesky Senior Analyst HE Global Content Strategy–Physical Sciences: Deborah Harden Content Management Manager of Content Development–HE Science: Matt Walker Development Editor: Edward Dodd Content Production Director, Production & Digital Studio, Science/ECS: Katherine Foley Producer–Science/ECS: Kristen Sanchez Managing Producer: Kristen Flathman Senior Content Producer: Martha Steele Content Producer, Media: Keri Rand Copyeditor: Carol Reitz Proofreader: Joanna Dinsmore Art and Design Director: Mark Ong/Side By Side Studios Interior/Cover Designer: Preston Thomas/Cadence Design Studio Product Management Product Manager Science–Physical Sciences: Darien Estes Product Marketing Senior Product Marketing Manager, Physical & Geological Sciences: Candice Madden Rights and Permissions Rights & Permissions Management: Ben Ferrini Photo Researcher: Paul James Tan
- 11. v About the Authors Randy Knight taught introductory physics for thirty-two years at Ohio State University and Cal- ifornia Polytechnic State University, where he is Professor Emeritus of Physics. Professor Knight received a PhD in physics from the University of California, Berkeley, and was a postdoctoral fellow at the Harvard-Smithsonian Center for Astrophysics before joining the faculty at Ohio State University. A growing awareness of the importance of research in physics education led first to Physics for Scientists and Engineers: A Strategic Approach and later to College Physics: A Strategic Approach. Professor Knight’s research interests are in the fields of laser spectroscopy and environmental science. When he’s not in front of a computer, you can find Randy hiking, traveling, playing the piano, or spending time with his wife Sally and their five cats. Brian Jones has won several teaching awards at Colorado State University during his thirty years teaching in the Department of Physics. His teaching focus in recent years has been the College Physics class, including writing problems for the MCAT exam and helping students review for this test. In 2011, Brian was awarded the Robert A. Millikan Medal of the American Association of Physics Teachers for his work as director of the Little Shop of Physics, a hands- on science outreach program. He is actively exploring the effectiveness of methods of informal science education and how to extend these lessons to the college classroom. Brian has been invited to give workshops on techniques of science instruction throughout the United States and in Belize, Chile, Ethiopia, Azerbaijan, Mexico, Slovenia, Norway, Namibia, and Uganda. Brian and his wife Carol have dozens of fruit trees and bushes in their yard, including an apple tree that was propagated from a tree in Isaac Newton’s garden. Stuart Field has been interested in science and technology his whole life. While in school he built telescopes, electronic circuits, and computers. After attending Stanford University, he earned a Ph.D. at the University of Chicago, where he studied the properties of materials at ultralow temperatures. After completing a postdoctoral position at the Massachusetts Institute of Technology, he held a faculty position at the University of Michigan. Currently at Colorado State University, Stuart teaches a variety of physics courses, including algebra-based introduc- tory physics, and was an early and enthusiastic adopter of Knight’s Physics for Scientists and Engineers. Stuart maintains an active research program in the area of superconductivity. Stuart enjoys Colorado’s great outdoors, where he is an avid mountain biker; he also plays in local ice hockey leagues. Contributing author Catherine Hirshfeld Crouch is Professor of Physics at Swarthmore College, where she has taught since 2003. Dr. Crouch’s work developing and evaluating cur- riculum for introductory physics for life science students has been used by faculty around the country and has been supported by the National Science Foundation. She earned her PhD at Harvard University in experimental condensed matter physics, and then remained at Harvard in a dual postdoctoral fellowship in materials physics and physics education with Eric Mazur, including developing and evaluating pedagogical best practices for undergraduate physics. She has published numerous peer-reviewed research articles in physics education and experimental physics, and has involved dozens of Swarthmore undergraduate students in her work. She is married to Andy Crouch and they have two young adult children, Timothy and Amy.
- 12. vi To the Student If you’re taking a physics course that uses this text, chances are that you intend a career in medicine or the life sciences. What are you expected to learn in physics that’s relevant to your future profession? Understanding physics is essential to a mastery of the life sciences for two key reasons: ■ ■ Physics and physical laws underlie all physiological pro- cesses, from the exchange of gases as you breathe to the propagation of nerve impulses. ■ ■ Many of the modern technologies used in biology and medicine, from fluorescent microscopy to radiation ther- apy, are based on physics concepts. Because of this critical role, physics is a major component of the MCAT. Biological systems are also physical systems, and a deep knowledge of biology requires understanding how the laws of physics apply to and sometimes constrain biological pro- cesses. One of our goals in this text is to build on the science you’ve learned in biology and chemistry to provide a solid understanding of the physical basis of biology and medicine. Another important goal is to help you develop your quan- titative reasoning skills. Quantitative reasoning is more than simply doing calculations. It is important to be able to do cal- culations, but our primary focus will be to discover and use patterns and relationships that occur in nature. Right away, in Chapter 1, we’ll present evidence showing that there’s a quantitative relationship between a mammal’s mass and its metabolic rate. That is, knowing the metabolic rate of a mouse allows you to predict the metabolic rate of an elephant. Mak- ing and testing predictions are at the heart of what science and medicine are all about. Physics, the most quantitative of the sciences, is a great place to practice these skills. Physics and biology are both sciences. They share many similarities, but learning physics requires a different approach than learning biology. In physics, exams will rarely test your ability to simply recall information. Instead, the emphasis will be on learning procedures and skills that, on exams, you will need to apply to new situations and new problems. You may be nervous about the amount of mathematics used in physics. This is common, but be reassured that you can do it! The math we’ll use is overwhelmingly the algebra, ge- ometry, and trigonometry you learned in high school.You may be a bit rusty (see Appendix A for a review of the math we’ll be using), and you almost certainly will understand this math better after using it in physics, but our many years of teaching experience find that nearly all students can handle the math. This text does use some calculus, and your instructor will decide how much or how little of that to include. Many of the ideas of physics—how fast things happen, how things accumulate—are expressed most naturally in the language of calculus. We’ll introduce the ideas gently and show you how calculus can be an important thinking and reasoning tool. In fact, many students find they understand calculus best after using it in physics. It’s important to become comfortable with calculus because it is increasingly used as a quantitative tool in the life sciences. How To Learn Physics There’s no single strategy for learning physics that works for everyone, but we can make a few suggestions that will help most students succeed: ■ ■ Read all of each chapter! This might seem obvious, but we know that many students focus their study on the worked examples. The worked examples are important and helpful, but to succeed on exams you will have to apply these ideas to completely new problems. To do so, you need to understand the underlying principles and logic that are explained in the body of the chapter. ■ ■ Use the chapter summaries. The chapter summaries are designed to help you see the big picture of how the pieces fit together. That said, the summaries are not a substitute for reading the chapter; their purpose is to help you consol- idate your knowledge after you’ve read the chapter. Notice that there are also part summaries at the end of each of the text’s six parts. ■ ■ Actively participate in class. Take notes, answer ques- tions, and participate in discussions. There is ample evidence that active participation is far more effective for learning science than passive listening. ■ ■ Apply what you’ve learned. Give adequate time and attention to the assigned homework questions and prob- lems. Much of your learning occurs while wrestling with problems. We encourage you to form a study group with two or three classmates. At the same time, make sure you fully understand how each problem is solved and are not simply borrowing someone else’s solution. ■ ■ Solve new problems as you study for exams. Questions and problems on physics exams will be entirely new prob- lems, not simply variations on problems you solved for homework. Your instructor wants you to demonstrate that you understand the physics by being able to apply it in new situations. Do review the solutions to worked examples and homework problems, focusing on the underlying rea- soning rather than the calculations, but don’t stop there. A much better use of time is to practice solving additional end-of-chapter problems while, as much as possible, refer- ring only to the chapter summaries. Our sincere wish is that you’ll find your study of physics to be a rewarding experience that helps you succeed in your chosen field by enhancing your understanding of biology and medi- cine. Many of our students report this was their experience!
- 13. vii To the Instructor University Physics for the Life Sciences has been written in response to the growing call for an introductory physics course explicitly designed for the needs and interests of life science students anticipating a career in biology, medicine, or a health-related field. The need for such a course has been recognized within the physics education community as well as by biological and medical professional societies. The Con- ference on Introductory Physics for the Life Sciences Report (American Association of Physics Teachers, 2014, available at compadre.org/ipls/) provides background information and makes many recommendations that have guided the develop- ment of this text. This new text is based on Knight Physics for Scientists and Engineers (4th edition, 2017) and Knight, Jones, Field College Physics (4th edition, 2019). As such, it is a research- based text based on decades of studies into how students learn physics and the challenges they face. It continues the engaging, student-oriented writing style for which the earlier books are known. At the same time, we have fully rethought the content, ordering, examples, and end-of-chapter problems to ensure that this text matches the needs of the intended audience. Objectives Our goals in writing this textbook have been: ■ ■ To produce a textbook that recognizes and meets the needs of students in the life sciences. ■ ■ To integrate proven techniques from physics education re- search and cognitive psychology into the classroom in a way that accommodates a range of teaching and learning styles. ■ ■ To help students develop conceptual and quantitative rea- soning skills that will be important in their professional lives. ■ ■ To prepare students to succeed on the Chemical and Physical Foundations of Biological Systems portion of the MCAT exam. Content and Organization Why develop a new textbook? What is needed to best meet the needs of life science students? The purpose of this text is to prepare students to grasp and apply physics content as need- ed to their discipline of choice—biology, biochemistry, and/ or health sciences. However, the introductory physics course taken by most life science students has for decades covered pretty much the same topics as those taught in the course for engineering and physics majors but with somewhat less math- ematics. Few of the examples or end-of-chapter problems deal with living systems. Such a course does not help life science students see the relevance of physics to their discipline. Many topics of biological importance are missing in a standard introductory physics textbook. These include viscos- ity, surface tension, diffusion, osmosis, and electrostatics in salt water. Applications such as imaging, whether in the form of fluorescence microscopy or scanning electron microscopy, are barely touched on. A physics course designed for life sci- ence students must be grounded in the fundamental laws of physics, a goal to which this text remains firmly committed. But how those laws are applied to the life sciences, and the examples that are explored, differ significantly from their ap- plication to engineering and physics. To endeavor to connect physics to the life sciences, we have added many topics that are important for biologists and physicians. To make time for these, we’ve scaled back some topics that are important for physicists and engineers but much less so for students in the life sciences. There’s less emphasis on standard force-and-motion problems; circular and rotational motion has been de-emphasized (and the text has been written to allow instructors to omit rotation entirely); some aspects of electricity and magnetism have been reduced; and relativity is omitted. After careful consideration, and con- sultation with experts in biology and physics education, we’ve made the choice that these topics are less relevant to the audi- ence than the new content that needed to be added. The most significant change is in the treatment of energy and thermodynamics. Energy and entropy are crucial to all living systems, and introductory physics could play a key role to help students understand these ideas. However, physicists and biologists approach these topics in very different ways. The standard physics approach that emphasizes conserva- tion of mechanical energy provides little insight into biological systems, where mechanical energy is almost never conserved. Further, biologists need to understand not how work is per- formed by a heat engine but how useful work can be extracted from a chemical reaction. Biologists describe energy use in terms of enthalpy and Gibbs free energy—concepts from chemistry—rather than heat and entropy. A presentation of energy and entropy must connect to and elucidate reaction dy- namics, enthalpy, and free energy if it is to help students see the relevance of physics to biology. Thus we’ve developed a new unit on energy and thermodynamics that provides a coherent development of en- ergy ideas, from work and kinetic energy through the laws of thermodynamics. Students bring a knowledge of atoms and molecules to the course, so a kinetic-theory perspective is emphasized. Molecular energy diagrams and the Boltzmann factor are used to understand what happens in a chemical re- action, and ideas about randomness lead not only to entropy but also to Gibbs free energy and what that tells us about the energetics of reactions. This text does use simple calculus, but more lightly than in the calculus-based introductory course for physicists and
- 14. engineers. Calculus is now a required course for biology majors at many universities, it is increasingly used as a quantitative analysis tool in biological research, and many medical schools expect at least a semester of calculus. Few results depend on calculus, and it can easily be sidestepped if an instructor de- sires an algebra-based course. Similarly, there are topics where the instructor could supplement the text with a somewhat more rigorous use of calculus if his or her students have the neces- sary math background. Although this text is oriented toward the life sciences, it assumes no background in biology on the part of the instructor. Examples and problems are self-contained. A basic familiarity with chemistry and chemical reactions is assumed. Key Features Many of the key features of this textbook are grounded in physics education research. ■ ■ Annotated figures, now seen in many textbooks, were introduced to physics in the first edition of Knight’s Physics for Scientists and Engineers. Research shows that the “instructor’s voice” greatly increases students’ ability to understand the many figures and graphs used in physics. ■ ■ Stop to Think Questions throughout the chapters are based on documented student misconceptions. ■ ■ NOTES throughout the chapters call students’ attention to concepts or procedures known to cause difficulty. ■ ■ Tactics Boxes and Problem-Solving Strategies help stu- dents develop good problem-solving skills. ■ ■ Chapter Summaries are explicitly hierarchical in design to help students connect the ideas and see the big picture. Instructor Resources A variety of resources are available to help instructors teach more effectively and efficiently. Most can be downloaded from the Instructor Resources area of MasteringTM Physics. ■ ■ Ready-To-Go Teaching Modules are an online instruc- tor’s guide. Each chapter contains background information on what is known from physics education research about student misconceptions and difficulties, suggested teaching strategies, suggested lecture demonstrations, and suggested pre- and post-class assignments. ■ ■ Mastering Physics is Pearson’s online homework system through which the instructor can assign pre-class reading quizzes, tutorials that help students solve a problem with hints and wrong-answer feedback, direct-measurement videos, and end-of-chapter questions and problems. Instructors can devise their own assignments or utilize pre-built assignments that have been designed with a good balance of problem types and difficulties. ■ ■ PowerPoint Lecture Slides can be modified by the instructor but provide an excellent starting point for class preparation. The lecture slides include QuickCheck questions. ■ ■ QuickCheck “Clicker Questions” are conceptual ques- tions, based on known student misconceptions. They are designed to be used as part of an active-learning teaching strategy. The Ready-To-Go teaching modules provide information on the effective use of QuickCheck questions. ■ ■ The Instructor’s Solution Manual is available in both Word and PDF formats. We do require that solutions for student use be posted only on a secure course website. viii To the Instructor
- 15. ix Instructional Package University Physics for the Life Sciences provides an integrated teaching and learning package of support material for students and instructors. NOTE For convenience, instructor supplements can be downloaded from the Instructor Resources area of Mastering Physics. Supplement Print Online Instructor or Student Supplement Description Mastering Physics with Pearson eText ✓ Instructor and Student Supplement This product features all of the resources of Mastering Physics in addition to the new Pearson eText 2.0. Now available on smartphones and tablets, Pearson eText 2.0 comprises the full text, including videos and other rich media. Instructor’s Solutions Manual ✓ Instructor Supplement This comprehensive solutions manual contains com- plete solutions to all end-of-chapter questions and problems. TestGen Test Bank ✓ Instructor Supplement The Test Bank contains more than 2,000 high-quality problems, with a range of multiple-choice, true/false, short answer, and regular homework-type questions. Test files are provided in both TestGen® and Word format. Instructor’s Resource Materials ✓ ✓ Instructor Supplement All art, photos, and tables from the book are available in JPEG format and as modifiable PowerPointsTM . In addition, instructors can access lecture outlines as well as “clicker” questions in PowerPoint format, editable content for key features, and all the instructor’s re- sources listed above. Ready-to-Go Teaching Modules ✓ Instructor Supplement Ready-to-Go Teaching Modules provide instructors with easy-to-use tools for teaching the toughest top- ics in physics. Created by the authors and designed to be used before, during, and after class, these modules demonstrate how to effectively use all the book, media, and assessment resources that accompany University Physics for the Life Sciences.
- 16. x Instructional Package Acknowledgments We have relied on conversations with and the written pub- lication of many members of the physics education com- munity and those involved in the Introductory Physics for Life Sciences movement. Those who may recognize their influence include the late Lillian McDermott and members of the Physics Education Research Group at the University of Washington, Edward “Joe” Redish and members of the Physics Education Research Group at the University of Mary- land, Ben Dreyfus, Ben Geller, Bob Hilborn, Dawn Meredith, and the late Steve Vogel. Ben Geller of Swarthmore College provided useful insights into teaching thermodynamics and contributed to the Ready-To-Go Teaching Modules. We are especially grateful to our contributing author Catherine Crouch at Swarthmore College for many new ideas and examples, based on her experience developing a course for life science majors, and for a detailed review of the entire manuscript. Thanks to Christopher Porter, The Ohio State University, for the difficult task of writing the Instructor’s Solutions Man- ual; to Charlie Hibbard for accuracy checking every figure and worked example in the text; to Elizabeth Holden, Univer- sity of Wisconsin-Platteville, for putting together the lecture slides; and to Jason Harlow, University of Toronto, for updat- ing the QuickCheck “clicker” questions. We especially want to thank Director HE Content Management Science & Health Sciences, Jeanne Zalesky; Product Manager Science–Physical Sciences, Darien Estes; Senior Analyst HE Global Content Strategy–Physical Sci- ences, Deborah Harden; Senior Development Editor, Alice Houston; Development Editor, Edward Dodd; Senior Content Producer, Martha Steele; Senior Associate Content Analyst Physical Science, Pan-Science, Harry Misthos; and all the other staff at Pearson for their enthusiasm and hard work on this project. It has been very much a team effort. Thanks to Margaret McConnell, Project Manager, and the composition team at Integra for the production of the text; Carol Reitz for her fastidious copyediting; Joanna Dinsmore for her precise proofreading; and Jan Troutt and Tim Brum- mett at Troutt Visual Services for their attention to detail in the rendering and revising of the art. And, last but not least, we each want to thank our wives for their encouragement and patience. Randy Knight California Polytechnic State University. Brian Jones Colorado State University. Stuart Field Colorado State University Reviewers and Classroom Testers Ward Beyermann, University of California–Riverside Jim Buchholz, California Baptist University David Buehrle, University of Maryland Robert Clare, University of California–Riverside Carl Covatto, Arizona State University Nicholas Darnton, Georgia Tech Jason Deibel, Wright State University Deborah Hemingway, University of Maryland David Joffe, Kennesaw State University Lisa Lapidus, Michigan State University Eric Rowley, Wright State University Josh Samani, University of California–Los Angeles Kazumi Tolich, University of Washington Luc Wille, Florida Atlantic University Xian Wu, University of Connecticut
- 17. xi Detailed Contents To the Student vi To the Instructor vii PART I Force and Motion 2 OVERVIEW The Science of Physics 3 Chapter 1 Physics for the Life Sciences 4 1.1 Why Physics? 5 1.2 Models and Modeling 7 1.3 Case Study: Modeling Diffusion 8 1.4 Proportional Reasoning: Scaling Laws in Biology 14 1.5 Where Do We Go from Here? 20 SUMMARY 21 QUESTIONS AND PROBLEMS 21 Chapter 2 Describing Motion 25 2.1 Motion Diagrams 26 2.2 Position, Time, and Displacement 27 2.3 Velocity 30 2.4 Acceleration 33 2.5 Motion Along a Straight Line 36 2.6 Units and Significant Figures 41 SUMMARY 46 QUESTIONS AND PROBLEMS 47 Chapter 3 Motion Along a Line 52 3.1 Uniform Motion 53 3.2 Instantaneous Velocity 56 3.3 Finding Position from Velocity 60 3.4 Constant Acceleration 62 3.5 Solving Kinematics Problems 67 3.6 Free Fall 69 3.7 Projectile Motion 72 3.8 Modeling a Changing Acceleration 76 SUMMARY 80 QUESTIONS AND PROBLEMS 81 Chapter 4 Force and Motion 90 4.1 Motion and Forces: Newton’s First Law 91 4.2 Force 92 4.3 A Short Catalog of Forces 95 4.4 What Do Forces Do? 98 4.5 Newton’s Second Law 100 4.6 Free-Body Diagrams 102 4.7 Working with Vectors 106 4.8 Using Newton’s Laws 111 SUMMARY 116 QUESTIONS AND PROBLEMS 117 Chapter 5 Interacting Systems 124 5.1 Systems and Interactions 125 5.2 Mass and Weight 125 5.3 Interactions with Surfaces 129 5.4 Drag 135 5.5 Springs and Elastic Forces 141 5.6 Newton’s Third Law 144 5.7 Newton’s Law of Gravity 150 SUMMARY 155 QUESTIONS AND PROBLEMS 156 Chapter 6 Equilibrium and Elasticity 163 6.1 Extended Objects 164 6.2 Torque 164 6.3 Gravitational Torque and the Center of Gravity 168 6.4 Static Equilibrium 172 6.5 Stability and Balance 176 6.6 Forces and Torques in the Body 179 6.7 Elasticity and Deformation 182 SUMMARY 190 QUESTIONS AND PROBLEMS 191 Chapter 7 Circular and Rotational Motion 199 7.1 Uniform Circular Motion 200 7.2 Dynamics of Uniform Circular Motion 202 7.3 The Rotation of a Rigid Body 208 7.4 Rotational Dynamics and Moment of Inertia 212 7.5 Rolling Motion 219 SUMMARY 222 QUESTIONS AND PROBLEMS 223
- 18. Chapter 8 Momentum 230 8.1 Momentum and Impulse 231 8.2 Conservation of Momentum 235 8.3 Collisions and Explosions 240 8.4 Angular Momentum 244 SUMMARY 248 QUESTIONS AND PROBLEMS 249 Chapter 9 Fluids 256 9.1 Properties of Fluids 257 9.2 Pressure 258 9.3 Buoyancy 266 9.4 Surface Tension and Capillary Action 272 9.5 Fluids in Motion 279 9.6 Ideal Fluid Dynamics 281 9.7 Viscous Fluid Dynamics 284 9.8 The Circulatory System 289 SUMMARY 295 QUESTIONS AND PROBLEMS 296 PART I SUMMARY Force and Motion 304 ONE STEP BEYOND Dark Matter and the Structure of the Universe 305 PART I PROBLEMS 306 PART II Energy and Thermodynamics 308 OVERVIEW Energy and Life 309 Chapter 10 Work and Energy 310 10.1 Energy Overview 311 10.2 Work and Kinetic Energy 313 10.3 Work Done by a Force That Changes 319 10.4 Dissipative Forces and Thermal Energy 322 10.5 Power and Efficiency 324 SUMMARY 330 QUESTIONS AND PROBLEMS 331 Chapter 11 Interactions and Potential Energy 337 11.1 Potential Energy 338 11.2 Conservation of Energy 340 11.3 Elastic Potential Energy 346 11.4 Energy Diagrams 350 11.5 Molecular Bonds and Chemical Energy 354 11.6 Connecting Potential Energy to Force 359 11.7 The Expanded Energy Model 360 11.8 Energy in the Body 362 SUMMARY 370 QUESTIONS AND PROBLEMS 371 Chapter 12 Thermodynamics 378 12.1 Heat and the First Law of Thermodynamics 379 12.2 Thermal Expansion 383 12.3 Specific Heat and Heat of Transformation 385 12.4 Calorimetry 390 12.5 Heat Transfer 393 12.6 The Ideal Gas: A Model System 398 12.7 Thermodynamics of Ideal Gases 404 12.8 Enthalpy 409 SUMMARY 414 QUESTIONS AND PROBLEMS 415 Chapter 13 Kinetic Theory 422 13.1 Connecting the Microscopic and the Macroscopic 423 13.2 Molecular Speeds and Collisions 423 13.3 The Kinetic Theory of Gases 425 13.4 Thermal Energy and Specific Heat 431 13.5 kBT and the Boltzmann Factor 437 13.6 Reaction Kinetics and Catalysis 441 13.7 Diffusion 445 SUMMARY 452 QUESTIONS AND PROBLEMS 453 xii Detailed Contents
- 19. Chapter 14 Entropy and Free Energy 459 14.1 Reversible and Irreversible Processes 460 14.2 Microstates, Multiplicity, and Entropy 463 14.3 Using Entropy 468 14.4 Spontaneity and Gibbs Free Energy 474 14.5 Doing Useful Work 479 14.6 Using Gibbs Free Energy 483 14.7 Mixing and Osmosis 485 SUMMARY 495 QUESTIONS AND PROBLEMS 496 PART II SUMMARY Energy and Thermodynamics 502 ONE STEP BEYOND Entropy and the Living World 503 PART II PROBLEMS 504 PART III Oscillations and Waves 506 OVERVIEW Motion That Repeats 507 Chapter 15 Oscillations 508 15.1 Simple Harmonic Motion 509 15.2 SHM and Circular Motion 513 15.3 Energy in SHM 516 15.4 Linear Restoring Forces 520 15.5 The Pendulum 525 15.6 Damped Oscillations 528 15.7 Driven Oscillations and Resonance 531 SUMMARY 534 QUESTIONS AND PROBLEMS 535 Chapter 16 Traveling Waves and Sound 543 16.1 An Introduction to Waves 544 16.2 Visualizing Wave Motion 546 16.3 Sinusoidal Waves 550 16.4 Sound and Light 555 16.5 Circular and Spherical Waves 560 16.6 Power, Intensity, and Decibels 562 16.7 The Doppler Effect 565 SUMMARY 570 QUESTIONS AND PROBLEMS 571 Chapter 17 Superposition and Standing Waves 578 17.1 The Principle of Superposition 579 17.2 Standing Waves 580 17.3 Standing Waves on a String 583 17.4 Standing Sound Waves 587 17.5 The Physics of Speech 591 17.6 Interference Along a Line 593 17.7 Interference in Two and Three Dimensions 599 17.8 Beats 603 SUMMARY 607 QUESTIONS AND PROBLEMS 608 PART III SUMMARY Oscillations and Waves 616 ONE STEP BEYOND Waves in the Earth and the Ocean 617 PART III PROBLEMS 618 PART IV Optics 620 OVERVIEW The Wave Model of Light 621 Chapter 18 Wave Optics 622 18.1 Models of Light 623 18.2 Thin-Film Interference 625 18.3 Double-Slit Interference 630 18.4 The Diffraction Grating 634 18.5 Single-Slit Diffraction 638 18.6 Circular-Aperture Diffraction 642 18.7 X Rays and X-Ray Diffraction 644 SUMMARY 648 QUESTIONS AND PROBLEMS 649 Detailed Contents xiii
- 20. 21.6 The Motion of a Charged Particle in an Electric Field 754 21.7 The Torque on a Dipole in an Electric Field 756 SUMMARY 761 QUESTIONS AND PROBLEMS 762 Chapter 22 Electric Potential 770 22.1 Electric Potential Energy 771 22.2 The Electric Potential 778 22.3 Calculating the Electric Potential 781 22.4 The Potential of a Continuous Distribution of Charge 787 22.5 Sources of Electric Potential 791 22.6 Connecting Potential and Field 793 22.7 The Electrocardiogram 797 SUMMARY 802 QUESTIONS AND PROBLEMS 803 Chapter 23 Biological Applications of Electric Fields and Potentials 811 23.1 Capacitance and Capacitors 812 23.2 Combinations of Capacitors 816 23.3 Dielectrics 819 23.4 Electrostatics in Salt Water 824 23.5 The Membrane Potential of a Cell 829 SUMMARY 835 QUESTIONS AND PROBLEMS 836 Chapter 24 Current and Resistance 842 24.1 A Model of Current 843 24.2 Defining Current 844 24.3 Batteries and emf 848 24.4 Resistance and Conductance 850 24.5 Ohm’s Law and Resistor Circuits 853 24.6 Energy and Power 856 24.7 Alternating Current 859 SUMMARY 864 QUESTIONS AND PROBLEMS 865 Chapter 25 Circuits 871 25.1 Circuit Elements and Diagrams 872 25.2 Using Kirchhoff’s Laws 873 25.3 Series and Parallel Circuits 876 25.4 Measuring Voltage and Current 881 25.5 More Complex Circuits 882 25.6 Electric Safety 885 25.7 RC Circuits 888 25.8 Electricity in the Nervous System 891 SUMMARY 898 QUESTIONS AND PROBLEMS 899 Chapter 19 Ray Optics 656 19.1 The Ray Model of Light 657 19.2 Reflection 659 19.3 Refraction 662 19.4 Image Formation by Refraction 667 19.5 Thin Lenses: Ray Tracing 668 19.6 The Thin-Lens Equation 674 19.7 Image Formation with Spherical Mirrors 677 19.8 Color and Dispersion 680 SUMMARY 685 QUESTIONS AND PROBLEMS 686 Chapter 20 Optical Instruments 692 20.1 Lenses in Combination 693 20.2 The Camera 694 20.3 The Human Eye 697 20.4 Magnifiers and Microscopes 702 20.5 The Resolution of Optical Instruments 706 20.6 Microscopy 710 SUMMARY 717 QUESTIONS AND PROBLEMS 718 PART IV SUMMARY Optics 724 ONE STEP BEYOND Phase-Contrast Microscopy 725 PART IV PROBLEMS 726 PART V Electricity and Magnetism 728 OVERVIEW Charges, Currents, and Fields 729 Chapter 21 Electric Forces and Fields 730 21.1 The Charge Model 731 21.2 A Microscopic Model of Charge 737 21.3 Coulomb’s Law 740 21.4 The Electric Field 744 21.5 The Electric Field of Multiple Charges 747 xiv Detailed Contents
- 21. 28.3 Photons 1000 28.4 Matter Waves 1003 28.5 Energy Is Quantized 1005 28.6 Energy Levels and Quantum Jumps 1007 28.7 The Uncertainty Principle 1009 28.8 Applications of Quantum Physics 1012 SUMMARY 1017 QUESTIONS AND PROBLEMS 1018 Chapter 29 Atoms and Molecules 1025 29.1 Spectroscopy 1026 29.2 Atoms 1027 29.3 The Hydrogen Atom 1033 29.4 Multi-electron Atoms 1037 29.5 Excited States and Spectra 1040 29.6 Molecules 1043 29.7 Fluorescence and Bioluminescence 1046 29.8 Stimulated Emission and Lasers 1047 SUMMARY 1051 QUESTIONS AND PROBLEMS 1052 Chapter 30 Nuclear Physics 1058 30.1 Nuclear Structure 1059 30.2 Nuclear Stability 1063 30.3 The Strong Force 1066 30.4 Radiation and Radioactivity 1069 30.5 Types of Nuclear Decay 1074 30.6 The Interaction of Ionizing Radiation with Matter 1079 30.7 Nuclear Medicine 1082 30.8 The Ultimate Building Blocks of Matter 1086 SUMMARY 1091 QUESTIONS AND PROBLEMS 1092 PART VI SUMMARY Modern Physics 1098 ONE STEP BEYOND The Physics of Very Cold Atoms 1099 PART VI PROBLEMS 1100 Appendix A Mathematics Review A-1 Appendix B Periodic Table of Elements A-5 Appendix C Studying for and Taking the MCAT Exam A-7 Appendix D Atomic and Nuclear Data A-11 Answers to Odd-Numbered Problems A-15 Credits C-1 Index I-1 Chapter 26 Magnetic Fields and Forces 907 26.1 Magnetism 908 26.2 The Magnetic Field of a Current 911 26.3 Magnetic Dipoles 915 26.4 The Magnetic Force on a Moving Charge 920 26.5 Magnetic Forces on Current- Carrying Wires 927 26.6 Forces and Torques on Magnetic Dipoles 929 26.7 Magnetic Resonance Imaging 932 SUMMARY 940 QUESTIONS AND PROBLEMS 941 Chapter 27 Electromagnetic Induction and Electromagnetic Waves 949 27.1 Induced Currents 950 27.2 Motional emf 951 27.3 Magnetic Flux and Lenz’s Law 955 27.4 Faraday’s Law 960 27.5 Induced Fields 963 27.6 Electromagnetic Waves 965 27.7 Polarization 968 27.8 The Interaction of Electromagnetic Waves with Matter 971 SUMMARY 977 QUESTIONS AND PROBLEMS 978 PART V SUMMARY Electricity and Magnetism 986 ONE STEP BEYOND The Greenhouse Effect and Global Warming 987 PART V PROBLEMS 988 PART VI Modern Physics 990 OVERVIEW New Ways of Looking at the World 991 Chapter 28 Quantum Physics 992 28.1 Physics at the Atomic Level 993 28.2 The Photoelectric Effect 994 Detailed Contents xv
- 22. Get Complete eBook Download Link below for instant download https://browsegrades.net/documents/2 86751/ebook-payment-link-for-instant- download-after-payment
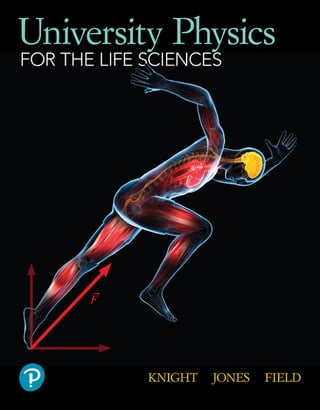
- 23. 2 Force and Motion P A R T I Elite athletes push the human body’s physical limits.What forces act on this sprinter as she accelerates? How much force can her muscles, tendons, and bones endure? How much air can flow into her lungs? How rapidly can blood be pumped through her veins?These are physics questions that help us understand human performance, questions we’ll address in Part I.
- 24. The Science of Physics Physics is the foundational science that underlies biology, chemistry, earth science, and all other fields that attempt to understand our natural world. Physicists couple careful experimentation with theoretical insights to build powerful and predictive models of how the world works. A key aspect of physics is that it is a unifying dis- cipline: A relatively small number of key concepts can explain a vast array of natural phenomena. In this text, we have organized the chapters into parts according to six of these unifying principles. Each of the six parts opens with an overview that gives you a look ahead, a glimpse of where your journey will take you in the next few chapters. It’s easy to lose sight of the big picture while you’re busy negotiating the terrain of each chapter. In Part I, the big picture is, in a word, change. Why Things Change Simple observations of the world around you show that most things change. Some changes, such as aging, are biological. Others, such as the burning of gasoline in your car, are chemical. In Part I, we will look at changes that involve motion of one form or another—from running and jumping to swimming microorganisms. There are two big questions we must tackle to study how things change by moving: ■ How do we describe motion? How should we measure or characterize the mo- tion if we want to analyze it quantitatively? ■ How do we explain motion? Why do objects have the particular motion they do? When you toss a ball upward, why does it go up and then come back down rather than keep going up? What are the “laws of nature” that allow us to predict an object’s motion? Two key concepts that will help answer these questions are force (the “cause”) and acceleration (the “effect”). Our basic tools will be three laws of motion worked out by Isaac Newton to relate force and motion. We will use Newton’s laws to explore a wide range of problems—from how a sprinter accelerates to how blood flows through the circulatory system. As you learn to solve problems dealing with motion, you will be learning techniques that you can apply throughout this text. Using Models Another key aspect of physics is the importance of models. Suppose we want to analyze a ball moving through the air. Is it necessary to analyze the way the atoms in the ball are connected? Or the details of how the ball is spinning? Or the small drag force it experiences as it moves? These are interesting questions, of course. But if our task is to understand the motion of the ball, we need to simplify! We can conduct a perfectly fine analysis of the ball’s motion by treating the ball as a single particle moving through the air. This is a model of the situation. A model is a simplified description of reality that is used to reduce the complexity of a problem so it can be analyzed and understood. Both physicists and biologists make extensive use of models to simplify complex situations, and in Part I you’ll begin to learn where and how models are employed and assessed. Learning how to simplify a situation is the essence of successful problem solving. 3 O V E R V I E W
- 25. LOOKING AHEAD Biology Is Subject to the Laws of Physics The rich diversity of life on our planet shares one thing in common: All living organisms are subject to the laws of physics. Physical laws, such as energy conservation and the laws of fluid flow, constrain what is possible for life, and life has responded to the challenge spec- tacularly. Physics also provides powerful tools for the life sciences, enabling us to image and measure cells and organisms ranging from viruses to humans. Biologists and physicians emphasize the importance of understanding how these tools work—particularly, recognizing when tools aren’t working correctly or won’t work effectively in a certain situation. Our goal in this text is to help you understand living systems and biomedical tools more deeply by exploring the physical mechanisms at work from large scales to small, from organisms down to molecules. This first chapter will introduce you to several key ideas. PHYSICS AND LIFE This scanning electron microscope image shows Salmonella bacteria (red) invading immune cells.You’ll learn about electron microscopes in Chapter 28. GOAL To understand some of the ways that physics and quantitative reasoning shed light on biological processes. Chapter Previews Each chapter starts with a preview outlining the major topics of the chapter and how they are relevant to the life sciences. Modeling A dialysis machine serves as an artificial kid- ney by having waste products diffuse through a membrane from blood into a dialysis liquid. Scaling If you know the metabolic rate of a hamster, you can calculate the metabolic rate of a horse—or of any other mammal. You’ll see how physicists model complex situations as we construct a model of dif fusion based on the idea of a random walk. You’ll learn how scaling laws connect many basic physiological processes to body size or body mass. Studies find that your understanding of a chapter is improved by knowing what key points to look for as you read. Magnetic resonance imaging (MRI) is just one of many ways that physics has contributed to biology and medicine.We’ll look at how images like this are created in Chapter 26. 1 Physics for the Life Sciences
- 26. 1.1 Why Physics? 5 1.1 Why Physics? Why take physics? Does knowing about projectiles, pendulums, or magnetic fields help you understand biological systems? Do doctors or microbiologists or ecologists think about or use the principles of physics? Actually, the answer to these questions is Yes. The existence of separate biology, chemistry, and physics departments may make it seem like these are distinct sci- ences, but that couldn’t be further from the truth. Our overarching goal in this text is to help you discover the central importance of physics to the life sciences. Biological systems are part of the physical world, and biological processes are physical pro- cesses, so the laws of physics can help you understand a great deal about biology, biochemistry, and medicine. Let’s look at some examples: Physics in biology The circulatory system, from the heart to the capillaries, is governed by fluid dynamics. You’ll study the physics of circulation in Chapter 9. Neurons signal each other via electric pulses that travel along axons. This is a topic we’ll visit in Chapter 25. The light-emitting properties of the green fluorescent protein are used to visualize cell structure. Fluorescence is covered in Chapter 29. Physics in biomechanics The physics of locomotion affects how fast an animal can run. Locomotion is the topic of several chapters in Part I. Physics helps us understand the limits of athletic performance. Chapter 11 looks at how the body uses energy. And physics explains how sap rises in trees through the xylem. This somewhat counter- intuitive fluid flow is described in Chapter 9. Physics in medicine Pulse oximetry measures blood oxygen with a clip-on device. Chapter 29 discusses how blood absorbs different colors of light. A patient prepares to have cancer treated by proton irradiation. Radiation and radia- tion therapy are topics in Chapter 30. Lasers are used for high-precision eye surgery of both the cornea and the retina. Chapter 20 covers the optics of the human eye.
- 27. 6 c h a p t e r 1 Physics for the Life Sciences These are among the many applications of physics to the life sciences that you’ll learn about in this book. That said, this is a physics textbook, not a biology textbook. We need to develop the underlying principles of physics before we can explore the ap- plications, so we will often start with rolling balls, oscillating springs, and other simple systems that illustrate the physical principles. Then, after laying the foundations, we will move on to see how these ideas apply to biology and medicine. As authors, our sincere hope is that this course will help you see why many things in biology happen as they do. Physics and Biology Physicists and biologists are scientists: They share many common views of what sci- ence is and how science operates. At the same time, as TABLE 1.1 illustrates, physicists and biologists often see the world in rather different ways. Your physics course will be less stressful and more productive if you’re aware of these differences. Physics tries to get at the essence of a process by identifying broad principles, such as energy conservation, and applying them to systems that have been greatly simplified by stripping away superfluous details. This approach is less common in biology, where systems often can’t be simplified without throwing out details that are essential to biological function. You may have taken biology courses in which you were expected to memorize a great deal of information; there’s much less to memorize in physics. Instead, most physicists agree that students demonstrate their understanding of physics by using general principles to solve unfamiliar problems. With that in mind, we can establish four large-scale goals for this text. By the end, you should be able to: • Recognize and use the principles of physics to explain physical phenomena. • Understand the importance of models in physics. • Reason quantitatively. • Apply the principles of physics to biological systems. The examples in this text will help you at each step along the way, and the end-of- chapter problems will provide many opportunities for practice. Mathematics Physics is the most quantitative of all the sciences. As physics has developed, the laws of physics have come to be stated as mathematical equations. These equations can be used to make quantitative, testable predictions about nature, whether it’s the orbit of a satellite or the pressure needed to pump blood through capillaries. This approach to science has proven to be extremely powerful; much of modern bio- medical technology—from electron microscopes to radiation therapy—depends on the equations of physics. But math is used in physics for more than simply doing calculations. The equa- tions of physics tell a story; they’re a shorthand way to describe how different concepts are related to one another. So, while we can use the ideal-gas law to calcu- late the pressure in a container of gas, it’s more useful to recognize that the pressure of a gas in a rigid container (one that has a constant volume) increases in exactly the same proportion as the absolute temperature. Consequently, doubling the tempera- ture causes the pressure to double. The ideal-gas law expresses a set of deep ideas about how gases behave. So, yes, we will often use equations to calculate values. But, more fundamentally, physics is about using math to reason and to analyze; that is, math is a thinking tool as much as it is a calculation tool. This may be a new way of using math for you, but—with some practice and experience—we think you’ll come to recognize the power of this way of thinking. The math used in this book is mostly math you already know: algebra, geometry, and some trigonometry. You might need some review (see Appendix A), but we’re confident that you can handle the math. Physics does use many symbols to represent TABLE 1.1 Characteristics of biology and physics Biology Physics Irreducibly complex systems Simple models More qualitative More quantitative Focus on specific examples Focus on broad principles Seeing the details A scanning electron microscope produces highly detailed images of biological structures only a few nanome- ters in size. The level of detail in this image of a budding HIV virus can provide new understanding of how the virus spreads and how it might be stopped. Physics has been at the forefront of developing a wide variety of imaging technologies.
- 28. 1.2 Models and Modeling 7 quantities, such as F for force, p for pressure, and E for energy, so many of our equations—like the ideal-gas law—are algebraic equations that show how quanti- ties are related to one another. It’s customary to use Greek letters to represent some quantities, but we’ll let you know what those letters are when we introduce them. We will, in some chapters, use a little bit of the calculus of derivatives and inte- grals. Don’t panic! Our interest, once again, is not so much in doing calculations as in understanding how different physical quantities are related to one another. And we’ll remind you, at the appropriate times, what derivatives and integrals are all about. In fact, most students feel that they come to understand calculus much better after study- ing physics because physics provides a natural context for illustrating why calculus is useful. 1.2 Models and Modeling The real world is messy and complicated. A well-established procedure in phys- ics is to brush aside many of the real-world details in order to discern patterns that occur over and over. For example, an object oscillating back and forth on a spring, a swinging pendulum, a vibrating guitar string, a sound wave, and an atom jiggling in a crystal seem very different—yet perhaps they are not really so different. Each is an example of a system oscillating around an equilibrium position. If we focus on understanding the properties of a very simple oscillating system, we’ll find that we understand quite a bit about the many real-world manifestations of oscillations. Stripping away the details to focus on essential features is a process called model ing. A model is a simplified picture of reality, but one that still captures the essence of what we want to study. Thus “mass on a spring” is a model of almost all oscillat- ing systems. Models allow us to make sense of complex situations by providing a framework for thinking about them. A memorable quote attributed to Albert Einstein is that physics “should be as simple as possible—but not simpler.” We want to use the simplest model that al- lows us to understand the phenomenon we’re studying, but we can’t make the model so simple that essential features of the phenomenon are lost. That’s somewhat of a problem in this text because understanding the physics of a biological system is not the same as understanding the biology. Our models of biological systems may seem to throw out much of the relevant biology, but keep in mind that our goal is to un- derstand the ways in which the physical properties of the system have a meaningful effect on aspects of the biology. We’ll develop and use many models throughout this textbook; they’ll be one of our most important thinking tools. These models will be of two types: • Descriptive models: What are the essential characteristics and properties of a phe- nomenon? How do we describe it in the simplest possible terms? For example, we will often model a cell as a water-filled sphere. This omits all the details about what’s inside, but for some purposes, such as estimating a cell’s mass or volume, we don’t need to know what’s inside. • Explanatory models: Why do things happen as they do? What causes what? Explanatory models have predictive power, allowing us to test—against experi- mental data—whether a model provides an adequate explanation of an observed phenomenon. A spring-like model of molecular bonds will allow us to explain many of the thermal properties of materials. Biologists also use models. A biological model, often qualitative rather than quantitative, is a simplified representation of the structure and function of a biologi- cal system or a biological process. The cell model is a simplified presentation of an immensely complex system, but it allows you to think logically about the key pieces and processes of a cell. Mice, fruit flies, and nematodes are important model organ isms that lend themselves to the study of particular biological questions without unnecessary complications. The eyes have it We study a model organ ism not to learn details about the organism but because the organism lends itself to the understanding of broad biological principles. Drosophila melanogaster, a common fruit fly, has provided immeasurable insights into genetics for more than a century.
- 29. 8 c h a p t e r 1 Physics for the Life Sciences At the same time, biology and medicine are becoming increasingly quantitative, with models more and more like those constructed by physicists. For example: • Mathematical models of enzyme kinetics provide quantitative predictions of complex biochemical pathways. • Neural network models improve our understanding of both real brains and artifi- cial intelligence. • Epidemiological models increase our knowledge of how disease spreads. • Global climate models that illustrate the earth’s climate and how it is changing depend on a complex interplay between living (e.g., photosynthesis) and nonliv- ing (e.g., solar radiation) processes. These models skip over many details in order to provide a big-picture understand- ing of the system. That’s the purpose of a model. This is not to say that details are unimportant—most scientists spend most of their careers studying the details—but that we don’t want to let the trees obscure our view of the forest. 1.3 Case Study: Modeling Diffusion Diffusion is one of the most important physical processes in biology. Oxygen diffuses from your lungs to your blood, and neurotransmitters diffuse across the synapses that connect one neuron to the next. We’ll study diffusion extensively in Chapter 13, but as a case study let’s see how a physicist might model diffusion and conclude when diffusion has biological significance. That is, our goal is to create a model that makes testable predictions of how far molecules can diffuse and how long it takes them to do so. NOTE ▶ The analysis in this section is more complex than you are expected to do on your own. However, it is expected that you will follow the reasoning and be able to answer questions about the procedure and the results. ◀ On a microscopic scale, molecules are constantly jostling around and colliding with one another, an atomic-level motion we’ll later associate with thermal energy. Any one molecule moves only a short distance before a collision sends it off in a dif- ferent direction. Its trajectory, if we could see it, might look something like FIGURE 1.1: lots of short, straight segments of apparently random lengths in what appear to be random directions. This is a chaotic and complex motion. The essence of modeling is to make simpli- fying assumptions, so how might we begin? The photographs in FIGURE 1.2 provide a clue. The left photo shows blue dye carefully deposited as a thin layer in a test tube of agar gel. The right photo is the same test tube one week later. It’s a slow process, but the dye has diffused both up and down in a symmetrical pattern. This suggests that we start not with the complex three-dimensional motion of a molecule but with the simpler case of diffusion along a one-dimensional linear axis. A sidewalk is a linear axis. Suppose you stand at one location on an east-west sidewalk and flip a coin. If it’s heads, you take one step to the east; if tails, one step to the west. Then you toss the coin again, and again, and again, each time randomly taking a step to the east or to the west. You would be engaged in what physicists and mathematicians call a one-dimensional random walk. Because collisions randomly redirect molecules, let’s see what we can learn by modeling molecular motion as a random walk. NOTE ▶ Variations on the random-walk model are used in applications rang- ing from protein folding and genetic drift to predicting share prices on the stock market. ◀ In particular, let’s imagine a molecule that starts at the origin, x = 0, and then takes a random walk along the x-axis. That is, the molecule, at regular intervals, FIGURE 1.1 The possible motion of one molecule as it collides with other molecules. FIGURE 1.2 Vertical diffusion of blue dye.
- 30. 1.3 Case Study: Modeling Diffusion 9 randomly takes a step whose length we’ll call d in either the +x@direction or the -x@direction. At each step there’s a 50% chance of going either way. To help make this clear, FIGURE 1.3 shows what might be the first 10 steps of the molecule. The first two steps are to the right, the third back to the left, and so on. The molecule seems to wander aimlessly—that’s the essence of random motion—and after 10 steps its position is x10 = -2d. Now diffusion involves not one molecule but many, so imagine that we have a very large number of molecules that each move in one dimension along the x-axis. Assume that each molecule starts at the origin and then undergoes a random walk. What can we say about the collective behavior of a large number of random-walking molecules? This is a problem that can be worked out exactly by using statistics, but the mathematical manipulations are a bit tricky. Instead, we’ll explore the model as a computer simulation, one that you could do yourself in a spreadsheet. Suppose you put a zero in a spreadsheet cell to show a molecule’s starting position. In the cell to the right, you use the spreadsheet’s random-number generator—a digital coin flip— to either add d to the initial position (a step to the right) or subtract d from the initial position (a step to the left). Then you do the same thing in the next cell to the right, and then the next cell to the right of that, and so on, each time adding or subtracting d from the previous cell with a 50% chance of each. The 101st cell will be the mol- ecule’s position after taking 100 random steps. Now you can add a second row of cells for a second molecule, a third row for a third molecule, and so on. It might take a big spreadsheet, but you can have as many molecules and as many steps as you wish. We’ve used exactly this procedure to simulate the random walks of 1000 molecules for 100 steps each. FIGURE 1.4 gives the result by using dots to show the final positions of each of these molecules. Note that all molecules must be at an even multiple of d after an even number of steps, so the possible positions after 100 steps are 0, {2d, {4d, and so on.You can see that the first molecule—the top row—ends up at x100 = 4d while the 453rd molecule in the 453rd row ends at x100 = 24d. x 1 2 4 8 3 5 6 7 9 10 -3d -2d -d 0 d 2d 3d Each step is equally likely to go right or left. Step number x10 is the molecule’s position at the end of step 10. x10 x4 x0 FIGURE 1.3 The first 10 steps of one possible random walk. -30d 1 1000 -20d -10d 0 Position on the x-axis after 100 steps Molecule number 10d 20d 30d Molecule 1 ends up at x100 = 4d. Molecule 453 ends up at x100 = 24d. FIGURE 1.4 The result of 1000 molecules following a random walk for 100 steps. The motion may be random, but the collective motion of many molecules reveals a pattern. Figure 1.4 shows that roughly half the molecules end up to the right of the origin and half to the left. That’s exactly what we would expect from randomly tak- ing steps to the right or left. Mathematically, we can say that the average position of all 1000 molecules is xavg = 0. That is, the center of this array of dots is at the origin. You can see this in the second photo of Figure 1.2: The dye has spread, but it has done so symmetrically so that the center of the dye, its average position, is still in the middle of the test tube. The center may not have moved, but the molecules are spreading out, which is exactly what diffusion is. However, the spreading is perhaps not as much as you might have expected. After 100 steps, a molecule could have reached x = 100d, but
- 31. 10 c h a p t e r 1 Physics for the Life Sciences you can see from Figure 1.4 that, in fact, most are less than 10d from the origin. Even the most adventuresome molecule—one in a thousand—has moved out a dis- tance of only 30d. A moment’s thought tells why. Any molecule’s position after 100 steps is determined by 100 coin tosses. Although you don’t expect to get exactly 50 heads and 50 tails, you do expect the number of heads to be fairly similar to the number of tails. In the same way, a molecule won’t move very far from the origin if the number of steps it takes to the right is fairly similar to the number it takes to the left. Reaching x = 100d would require tossing 100 heads in a row, an outcome that is extraordinarily unlikely. We can analyze the 1000 molecular positions in Figure 1.4 by creating the histogram of FIGURE 1.5. A histogram is a bar chart that shows how many molecules end up at each position. The histogram is a bell curve, showing that x100 = 0 is the most likely final position, occurring 80 times out of the 1000 molecules, and that final positions becomes less likely as you move away from the origin. The computer- simulation bell curve is a bit ragged with only 1000 molecules, but, as you can imagine, it would become a very smooth curve if we could run the simulation with billions of molecules. Measuring the Spread Due to Diffusion Diffusing molecules spread out, and that’s exactly what the molecules in our model are doing. How can we measure this? What quantity describes the amount of spread- ing? Averaging the positions of all the molecules results in zero, as we’ve seen, so the average position doesn’t tell us anything about spreading.You might think of averag- ing the absolute values of the positions; those are all positive, and their average— which is not zero—really would give some information about spreading. However, it turns out that working with absolute values presents mathematical difficulties. Rather than averaging absolute values, let’s average the squares of the positions of each of the molecules. That is, we square the positions of all 1000 molecules, add the squares, and then divide by 1000 to find the average. We’ll use the nota- tion (x2 )avg to indicate this average of the squares of the positions. Squares are positive, so (x2 )avg is not zero. But is it useful? To find out, FIGURE 1.6 graphs x2 during the 100 steps of our computer simulation both for one molecule and for (x2 )avg when averaged over all 1000 molecules. The graph for one molecule is always positive, as it must be, but not very useful. It looks like the molecule managed to walk out to x = {8d in 33 steps, making x2 = 64d2 , then returned to the origin after 43 steps, got as far as x = {9d after 87 and 89 steps, but ended back at x = {6d. All in all, it’s pretty much what you might expect for one molecule engaged in a random walk. But averaging the squares of the positions of a large number of molecules tells a different story. You can see that (x2 )avg increases linearly with the number of steps, and the average becomes a better and better straight line if we increase the number of molecules in the average. Furthermore, it appears that (x2 )avg ≈ 50d2 after 50 steps and (x2 )avg ≈ 100d2 after 100 steps. That is, our computer simulation suggests that (x2 )avg = nd2 after n steps. And, indeed, a rigorous statistical analysis proves that this is true. The square root of (x2 )avg is called the root-mean-square distance xrms, often called the rms distance. Taking the square root of (x2 )avg = nd2 gives xrms = 2(x2 )avg = 1nd (1.1) The root mean square—the square root of the mean (i.e., the average) of the squares—is a useful way of describing the spread of a set of values that are sym- metrical about zero. We’ll revisit this concept at several points throughout this text. For our random-walking molecules, the root-mean-square distance xrms after n steps is simply the step size d multiplied by the square root of n. Thus xrms = 116d = 4d after 16 steps and xrms = 1100d = 10d after 100 steps, as our simulations showed. 0 20 40 60 80 100 100d2 80d2 60d2 40d2 20d2 0 Number of steps (x2 )avg for 1000 molecules x2 of one molecule x2 FIGURE 1.6 A graph showing how x2 changes for one molecule and, on average, for 1000 molecules during the first 100 steps of a random walk. -30d 80 60 40 20 0 -20d -10d 0 Position on the x-axis after 100 steps Number of occurrences 10d 20d 30d The most likely final position is x100 = 0. xrms FIGURE 1.5 A histogram of the 1000 molecular positions in Figure 1.4.
- 32. 1.3 Case Study: Modeling Diffusion 11 The root-mean-square distance is shown on the histogram of Figure 1.5, and you can see that xrms is a reasonably good answer to the question: What is the typical distance that the molecules have spread after n steps? It can be shown, using the tools of probability and statistics, that xrms is the stan dard deviation of the histogram, a term you may have encountered in statistics. One can show that, on average, 68% of all the molecules have positions between -xrms and +xrms, while only 5% have traveled farther than 2xrms. Of our 1000 molecules that took 100 steps and have xrms = 10d, we would expect 680 to have traveled no farther than 10d from the origin and only 50 to have exceeded 20d. This is a good description of the results shown in Figures 1.4 and 1.5. Thus xrms appears to be quite a good indicator of about how much spreading has occurred after n steps. We can say that xrms is the diffusion distance. What is the diffusion distance as a fraction of the maximum possible travel distance after 102 , 106 , and 1012 steps? PREPARE The maximum possible travel distance increases linearly with the number of steps n, but the diffusion distance increases more slowly, with the square root of n. SOLVE If a molecule moves in the same direction for every step, it will travel distance xmax = nd in n steps. This is the maximum possible travel distance. The diffusion distance is xdiff = xrms = 1nd. Thus xdiff xmax = 1nd nd = 1 1n Diffusive spreading For the different values of n we can compute xdiff xmax = • 10-1 for n = 102 10-3 for n = 106 10-6 for n = 1012 ASSESS Compared to how far a molecule could travel, the diffu- sion distance rapidly decreases as the number of steps increases. Diffusion is 10% of the maximum distance after 100 steps but only one-millionth of the maximum distance after 1012 steps, a number that is comparable to the number of collisions a molecule undergoes each second. Having lots of collisions does not mean rapid spreading. EXAMPLE 1.1 NOTE ▶ Worked examples in this text will follow a three-part problem-solving strategy: Prepare, Solve, and Assess. These examples are intended to illustrate good problem-solving procedures, and we strongly encourage you to use the same approach in your own work. We’ll look at this problem-solving strategy in more detail in Chapter 3. ◀ Connecting to the Real World Our analysis of the random walk has revealed some interesting similarities to what is known about diffusion, but how can we judge whether this model is an accurate description of diffusion? What quantitative prediction can we make to connect this model to what happens in the physical world? Two things that we can easily measure are distance and time, so let’s work out the relationship between the diffusion distance and the length of time that the sys- tem spends diffusing. We can then compare measurements to the predictions of this model to see how good the model is. First, let’s represent the time interval between steps of the random walk by the symbol ∆t, where ∆ is an uppercase Greek delta. As you’ll see in Chapter 2, we use the symbol t to represent a specific instant of time and ∆t to represent an interval of time. If the molecules start moving at time t = 0, then at a later time t they will have taken n = t/∆t steps. If, for example, a step is taken every ∆t = 1 10 s, there will have been 100 steps at t = 10 s. We can write xrms in terms of t and ∆t as xrms = 1nd = A t ∆t d = B td2 ∆t = 1vdt For the last step we used the fact that d/∆t = distance/time, as in miles per hour or meters per second, is the molecule’s speed. Throughout this text we’ll use the symbol v (from velocity) to represent speed, with v = d/∆t.
- 33. 12 c h a p t e r 1 Physics for the Life Sciences Let’s define the diffusion coefficient D to be D = 1 2 d2 ∆t = 1 2 vd (1.2) The diffusion coefficient connects our model to the physical world because it de- pends on the speed v of molecules and the distance d traveled between collisions. A factor of 1 2 is included in the definition to avoid a factor of 2 in an important equation that you’ll meet in Chapter 13. Thus our model predicts that the root-mean-square distance should increase with time as xrms = 22Dt (1.3) The real world is three dimensional, not a straight line. That turns out to be an easy extension of our model. Suppose each step is {d along the x-axis and {d along the y-axis and {d along the z-axis. In this 3D world, a molecule’s distance from the starting point is r = 2x2 + y2 + z2 . Averaging the squares, as we did above, gives (r2 )avg = (x2 )avg + (y2 )avg + (z2 )avg The x-axis does not differ from the y-axis or the z-axis, so averages should be the same along each axis. Thus (r2 )avg = (x2 )avg + (y2 )avg + (z2 )avg = 2Dt + 2Dt + 2Dt = 6Dt (1.4) If we define the three-dimensional root-mean-square distance as rrms = 2(r2 )avg , then rrms = 26Dt (1.5) This is our model’s prediction for how far molecules will diffuse in three dimensions in time t. Equation 1.5 is a quantitative, testable prediction of our model. It says that the distance molecules diffuse should increase with the square root of the elapsed time. This is not what your intuition suggests. If you walk or run or drive at a steady speed, you go twice as far in twice the time. But because 22 = 1.41, our prediction is that molecules will diffuse only 1.41 times as far in 20 s as they do in 10 s. It will take 40 s to diffuse twice as far as in 10 s. In fact, this prediction is confirmed by count- less experiments. To really test our model, we would also like to predict a numerical value for the diffusion coefficient D. What do we know that will help us at least estimate D? Estimating is a valuable scientific skill, one we’ll spend a fair amount of time on in this text, so let’s look at how to approach an estimation. Our random-walk model is a simplification of the idea that molecules are constantly colliding and changing direction, so let’s think about what is known about molecular motions and collisions. For one thing, we can infer that molecules in a liquid are pretty much touching each other. Liquids, unlike gases, are essentially incompressible—the molecules can’t get any closer together. The distance a molecule can move between collisions is at most the diameter of a molecule, probably less. You learned in chem- istry that the size of small molecules such as oxygen and water is about 0.1 nm, where 1 nm = 1 nanometer = 10-9 m. So let’s estimate that the random-walk step size, the distance between collisions, is d ≈ 0.05 nm = 5 * 10-11 m. How fast do molecules move? We can estimate molecular speeds by thinking about sound waves. Sound travels through a medium because the molecules run into each other, propagating a sound wave forward. We’ll look at the details when we study sound, but it seems reasonable that the sound speed is probably fairly similar to the speed of a molecule. We could look up the speed of sound in a reference book or on the Internet, but we can also estimate it from an experience that many of you have had.You may have learned that the distance to a lightning strike can be determined by counting the seconds from seeing the lightning to hearing the thunder, then dividing by 5 to get the distance in miles. The implication is that the speed of sound is about Crossing the gap Nerve impulses travel- ing from neuron to neuron have to cross the synaptic cleft between the axon terminal of one neuron and the dendrite receptor of the next. When the sending terminal on the axon is electrically activated, it releases chemi- cals called neurotransmitters that simply diffuse across the gap. The gap is so narrow, approximately 20 nm in width, that the diffusing neurotransmitters reach the other side and stimulate a response in less than a microsecond.
- 34. 1.3 Case Study: Modeling Diffusion 13 1 5 mi/s. A mile is about 1600 m, so 1 5 mi/s = 320 m/s. Consequently, our second esti- mate is that the speed with which molecules travel between collisions is v ≈ 320 m/s. We have assumed that molecular speeds in liquids are not greatly different from molecular speeds in air. You’ll learn when we study waves that the speed of sound in water is about four times the speed in air. That’s a big difference for some applications, such as whether ultrasound imaging works better in air or water, but it’s not a large discrepancy when our goal is merely a rough estimate of the diffusion coefficient. With that, we can now predict that the diffusion coefficient for small molecules diffusing through a liquid like water should be approximately D = 1 2 vd ≈ 1 2 (320 m/s)(5.0 * 10-11 m) ≈ 8 * 10-9 m2 /s (1.6) Notice the rather unusual units for a diffusion coefficient; these arise because of the square-root relationship between diffusion distance and time. Also notice that the value is given to only one significant figure because the num- bers that went into this calculation are only estimates; they are not precisely known. This is called an order-of-magnitude estimate, meaning that we wouldn’t be sur- prised to be off by a factor of three or four but that our estimate is almost certainly within a factor of ten of the real value. That may seem a poor outcome, but initially we had no idea what the value of a diffusion coefficient might be. To have deter- mined by simple reasoning that D is approximately 10-8 m2 /s (rounding the value of D in Equation 1.6 to the nearest power of 10), rather than 10-10 m2 /s or 10-6 m2 /s, is an important increase in our understanding of diffusion, one that can be improved only by careful laboratory measurements. Estimate how long it takes small molecules such as oxygen to diffuse 10 mm (about the diameter of a cell) and 10 cm (roughly the size of a small animal) through water, the primary component of intercellularfluid.Notethat 1 mm = 1 micrometer or 1 micron = 10-6 m. PREPARE The root-mean-square distance rrms tells us how far diffusion carries the molecules in time t. We’ll use our estimated D ≈ 8 * 10-9 m2 /s as the diffusion coefficient. SOLVE The root-mean-square distance is rrms = 26Dt. Solving for the time needed for molecules to diffuse distance rrms gives t = (rrms)2 6D Diffusing across a cell requires rrms = 10 mm = 1 * 10-5 m. Thus tcell = (1.0 * 10-5 m)2 6(8 * 10-9 m2 /s) ≈ 2 * 10-3 s = 2 ms Diffusion times But diffusing through a small animal requires rrms = 10 cm = 0.1 m. In this case, tcell = (0.1 m)2 6(8 * 10-9 m2 /s) ≈ 2 * 105 s ≈ 2 days ASSESS Diffusion of oxygen across a cellular distance of a few mm is very rapid. Oxygen in your blood is easily transported throughout cells by diffusion as long as no cell is more than a couple of cells away from a capillary. Unicellular organisms such as amoebas can use diffusion to passively harvest the oxy- gen from their environment. But diffusion across larger distances is impractically slow. A small animal, or one of your organs, is roughly 104 times larger than a cell. Because diffusion times de- pend on the square of the distance, diffusion across 10 cm takes 108 times as long as diffusion across a cell. The second photo in Figure 1.2, the dye diffusion, was taken a week after the experi- ment started, and the diffusion distance is only a few centimeters. EXAMPLE 1.2 We should feel pretty satisfied. Starting with a very simplified idea about what hap- pens in molecular collisions and using just a few basic ideas about the physical world, we’ve put together a model of diffusion that has strong explanatory power. Our esti- mated value of D for small molecules turns out to be about a factor of four too large, as you’ll see in Chapter 13, but, as we might say colloquially, it’s certainly in the ballpark. Furthermore, this result tells us why an amoeba doesn’t have lungs. A creature of its size doesn’t need them! But you do. Diffusion is inadequate to transport oxygen and other molecules throughout an organism of your size, so you need an actively powered (i.e., using metabolic energy) circulatory system. The laws of physics have real consequences for organisms.
- 35. 14 c h a p t e r 1 Physics for the Life Sciences Our goal in this section was to demonstrate how physicists model a complex situ- ation and how physics can increase your understanding of biological systems. The mathematics we used was not especially complex, but we certainly emphasized quan- titative reasoning. Graphs were also a useful tool to shape our thinking. These are the types of reasoning skills that we will help you develop throughout this textbook. NOTE ▶ Each chapter will have several Stop to Think questions. They are designed to see if you have processed and understood what you’ve read. The answers are given at the end of the chapter, but you should make a serious effort to think about these questions before turning to the answers. They are an impor- tant part of learning. A wrong answer should spur you to review the section before going on. ◀ 1.4 Proportional Reasoning: Scaling Laws in Biology The smallest terrestrial mammal is the Etruscan shrew, with a length of 4 cm and mass of a mere 2 g. The largest is the 7-m-long, 6000 kg African elephant. If we were to enlarge a shrew by a factor of 175, giving it a 7 m length, would it look pretty much like a furry elephant? We can’t carry out the experiment, but we can simulate it with photographs. FIGURE 1.7 shows a shrew and an elephant with, at least photographically, the same body length. We could say that the shrew has been scaled to the size of the elephant. In some regards, the answer to our question isYes. The scaled-up shrew is a bit chub- bier than the elephant, but the height, the ear size, and other features are all about the same. But what’s with the legs? Compared to the elephant, the legs of the scaled-up shrew are twigs rather than tree trunks. This is not an accident; there are underlying physical reasons why large animals are not just scaled-up versions of small animals. It all has to do with scaling laws, which are regularities in how the physical characteristics of organisms—from bone size to heart rate—depend on the organism’s size. Proportionality You often hear it said that one quantity is proportional to another. What does this mean? We say that y is linearly proportional to x if y = Cx (1.7) where C is a constant called the proportionality constant. This is sometimes written y ∝ x, where the symbol ∝ means “is proportional to.” As FIGURE 1.8 shows, a graph of y versus x is a straight line passing through the origin with slope C. NOTE ▶ A graph of “a versus b” means that a is graphed on the vertical axis and b on the horizontal axis. Saying “graph a versus b” is a shorthand way of say- ing “graph a as a function of b,” indicating that b is the independent variable on the horizontal axis. ◀ For example, the mass of an object is related to its volume by m = rV, where r (the Greek letter rho—we use a lot of Greek letters in physics so as to have enough The line passes through the origin. The slope of the line is C. 0 2 4 4C 2C 0 y x FIGURE 1.8 A graph showing linear proportionality. FIGURE 1.7 Comparing a shrew and an elephant. STOP TO THINK 1.1 Our estimate of the diffusion coefficient D is for the diffu- sion of small molecules through water. The same type of reasoning predicts that the diffusion coefficient for diffusion through air is _______ the diffusion coefficient for diffusion through water. A. Smaller than B. About the same as C. Larger than
- 36. 1.4 Proportional Reasoning: Scaling Laws in Biology 15 symbols for our needs) is the object’s density. Thus mass is linearly proportional to volume with, in this case, density being the proportionality constant. Proportionality allows us to use ratios to draw conclusions without needing to know the proportionality constant. Suppose x has an initial value x1, and thus y has the initial value y1 = Cx1. If we change x to x2, then y changes to y2 = Cx2. The ratio of y2 to y1 is y2 y1 = Cx2 Cx1 = x2 x1 (1.8) That is, the ratio of y2 to y1 is exactly the same as the ratio of x2 to x1, meaning that x and y scale up or down by the same factor. If you double an object’s volume, its mass doubles. If you decrease an object’s volume by a factor of three, its mass decreases by a factor of three. This type of reasoning is called ratio reasoning. NOTE ▶ Linear proportionality is more specific than a linear relationship. The linear relationship y = Cx + B also has a straight-line graph, but the graph has a y-intercept B; the line does not pass through the origin. Ratio reasoning does not work with a linear relationship because the constants don’t cancel. A linear pro- portionality is a special case of a linear relationship whose graph passes through the origin (B = 0). ◀ Now consider the surface area of a sphere: A = 4pr2 . This is also a proportion- ality, but in this case we would say, “The area is proportional to the square of the radius” or A ∝ r2 . Proportionalities don’t have to be linear (i.e., dependent on the first power of x); any relationship in which one quantity is a constant times another quantity is a proportionality. And ratio reasoning works with any proportionality because the constant cancels. A spherical type A cell has a surface area of 2 mm2 . The diameter of a type B cell is three times that of a type A cell. What is the surface area of a type B cell? PREPARE We could use the surface-area formula to find the radius of a type A cell, multiple it by 3, and then calculate the surface area of a type B cell. That’s a lot of calculation, though, with the possibility for making mistakes. Instead, let’s use ratio reasoning. SOLVE The ratio of the surface areas is AB AA = 4pr 2 B 4pr 2 A = a rB rA b 2 The surface area of a cell The proportionality constant 4p cancels, and we find that the ratio of the areas is the square of the ratio of the radii. If rB /rA = 3, then AB = 32 * AA = 9AA = 18 mm2 . ASSESS This is what we mean by reasoning with math rather than seeing math as simply a calculation tool. With practice, this is a problem you can solve in your head! “Let’s see, area is proportional to the square of the radius. If the radius in- creases by a factor of 3, the area will increase by a factor of 32 = 9.” EXAMPLE 1.3 For a person or object that moves at a steady speed, the time needed to travel a specified distance is inversely proportional to the speed; that is, ∆t ∝ 1/v, where v is the symbol for speed. Angela gets to work in 15 minutes if she walks at a steady speed. How long will it take if she rides her bicycle and cycles three times as fast as she walks? PREPARE We don’t know either the distance to work or Angela’s walking speed, but we don’t need either if we use ratio reasoning. SOLVE Because ∆t = C/v, with C being some unknown con- stant, we have ∆tcycle ∆twalk = C/vcycle C/vwalk = vwalk vcycle Time to work The ratio of the times is the inverse of the ratio of the speeds. We know that vcycle = 3vwalk, so ∆tcycle ∆twalk = vwalk 3vwalk = 1 3 Thus ∆tcycle = 1 3 ∆twalk = 5 min. ASSESS It makes sense that it takes one-third as long if Angela goes three times as fast. EXAMPLE 1.4
- 37. 16 c h a p t e r 1 Physics for the Life Sciences Proportionality helps us understand why the elephant’s legs are much thicker than the legs of the scaled-up shrew. An object’s volume V depends on the cube of some linear dimension l, such as a radius or an edge length. The exact formula for volume depends on the specific shape, but for any object—including animals—V ∝ l3 . For a given density, mass is proportional to volume and, because all mammals have about the same density, mass also obeys M ∝ l3 . We say that mass scales with the cube of the linear dimension. If we scale up an animal by a factor of five, quintupling the size of the animal in all three dimensions, its mass increases by a factor of 53 = 125. An animal’s legs have to support its weight. An animal that’s 125 times more massive needs bones that are 125 times stronger so as not to break under the load. You will see in Chapter 6 that the strength of bones is proportional to their cross- section area, but that’s a problem for large animals. Areas, whether those of circles, squares, or any other shape, scale as the square of the linear dimension: A ∝ l2 . In scaling up an animal by a factor of five, areas and bone strength increase by only a factor of 52 = 25. In other words, bone strength is not keeping up with the animal’s increase in weight. And to get from a shrew to an elephant we have to scale not by a factor of five but by a factor of 175! An animal’s mass scales as l3 , where l is its linear dimension, but its bone strength scales as only l2 . The weight of an animal that is simply scaled up would quickly out- strip its leg bones’ ability to support that weight, so a simple scaling up in size doesn’t work. To support the weight, the cross-section area of the bones must also scale as l3 . This means that the bone diameter has to scale as l3/2 , faster than the rate at which the length of the animal increases. That’s why the diameter of the elephant’s tree-trunk-like legs is a much larger fraction of its body length than the twig-like legs of the shrew. Allometry It may seem unlikely that there are mathematically expressible “laws of biology.” The great core principles of biology—such as evolution, structure-function relation- ships, and the connections between an organism and its genetic information—don’t readily lend themselves to being expressed in equations. But consider FIGURE 1.9, a graph of basal metabolic rate (the rate at which a resting animal uses energy, ab- breviated BMR) versus mass m for a large number of mammals, ranging from the smallest (the 2 g Etruscan shrew) to the largest (a 1.5 * 108 g blue whale). Recall 100 101 102 103 104 105 106 107 108 109 105 104 103 102 101 100 10-1 10-2 BMR (W) m (g) Blue whale Moose Human Rabbit Etruscan shrew Trend line slope = 0.76 FIGURE 1.9 Basal metabolic rate as a function of mass for mammals.
- 38. 1.4 Proportional Reasoning: Scaling Laws in Biology 17 that “BMR versus mass” graphs BMR on the vertical axis and mass—the indepen- dent variable—on the horizontal axis. NOTE ▶ Notice that graph axis labels tell us the units that are being used. Mass is measured in grams (g), while BMR is measured in watts (W). Metabolic rate is energy used per second, and the watt—just as used with your appliances to show the rate of energy use—is the metric unit. ◀ The data points fall just about perfectly along a straight line, suggesting that there is some kind of law relating a mammal’s BMR to its mass. The mass of the blue whale is nearly 108 times larger than the mass of the shrew, so this apparent law holds over an extremely wide range of masses. When two numbers differ by a factor of L10, we say that they differ by an order of magnitude. Thus the range of masses spans eight orders of magnitude. Similarly, the BMR values shown on the vertical axis span about six orders of magnitude. But you may have noticed that Figure 1.9 is not the type of graph you usually work with. You’re familiar with graphs where the tick marks on the axis represent constant intervals. That is, the tick marks might be labeled 0, 1, 2, 3, cwith an interval of 1. Or 0, 20, 40, 60, cwith an interval of 20. But the horizontal-axis tick marks in Figure 1.9 are labeled 100 , 101 , 102 , 103 , c. That is, each tick mark is a constant factor of 10 larger than the preceding tick mark. The same is true on the vertical axis. Figure 1.9, called a log-log graph, graphs not BMR versus mass but the logarithm of BMR versus the logarithm of mass—that is, log(BMR) versus log(m). Log-log graphs are widely used in science, especially—as here—when the data span many orders of magnitude. Data that fall on a straight line on a log-log graph are said to follow a scaling law. We need to review some properties of logarithms to see what this means. Any positive number a can be written as a power of 10 in the form a = 10b . Simple cases are 1 = 100 , 10 = 101 , and 100 = 102 . You can use your calculator to find that 210 = 3.16, so 3.16 = 101/2 = 100.50 . We define the logarithm of a as follows: If a = 10b then log(a) = b (1.9) Thus log(1) = 0, log(10) = 1, log(100) = 2, and log(3.16) = 0.50. You can use your calculator to find that log(25) = 1.40, which means that 25 = 101.40 . NOTE ▶ We’re using base10 logarithms, also called common logarithms. You may also be familiar with natural logarithms. We’ll see those later, but data anal- ysis is usually done with common logarithms. ◀ Because 10b # 10d = 10b+d , you should be able to convince yourself that log(a # c) = log(a) + log(c). This is an important property of logarithms. And be- cause an is a # a # a # g # a, multiplied n times, the logarithm of an is log(a) added n times; that is, log(an ) = nlog(a). Log-log graphs are widely used in science. It’s important to know how to read them, but the axis labels can be confusing. FIGURE 1.10 shows a portion of the hori- zontal axis from Figure 1.9 with two different ways of labeling the axis. The labels above the axis—powers of 10—are what Figure 1.9 shows, but they are not what is graphed. What’s actually graphed is the logarithms of these powers of 10, which are the labels shown below the axis. That is, the tick mark labeled 103 actually rep- resents the value 3.0, the logarithm of the label. So the axis really is increasing 0, 1, 2, 3, c, with a constant interval between the tick marks, but the tick marks are labeled, instead, with the powers of 10. Notice how the midpoints between the tick marks are about 3, 30, 300, c. This follows from the fact that their logarithms are about 0.5, 1.5, 2.5,c. This method of labeling, while potentially confusing until you get used to it, is quite useful. The top labels in Figure 1.10 tell the values of the masses, which is what These axis labels show the masses whose logarithms are being graphed. Because log(3) ≈ 0.5, the midpoint between the tick marks is ≈ 3 * 10n . These axis labels show the logarithms of the masses. 5.0 … 105 4.0 104 3.0 log[m (g)] 103 m (g) 2.0 102 1.0 101 3 30 0.0 100 FIGURE 1.10 Two ways to label a logarithmic axis.
- 39. 18 c h a p t e r 1 Physics for the Life Sciences you really want to know. The bottom labels tell only the logarithms of the masses, which are harder to interpret. Returning to Figure 1.9, we see that the trend line—the straight line that best “fits” the data—has a slope of 0.76. Slope, you will recall, is the rise-over-run ratio. But the rise and run of what? Because this is a graph of log(BMR) versus log(m), the slope is measured as the “rise” in log(BMR) divided by the “run” in log(m). That is, the slope is determined from the unseen logarithm labels 0, 1, 2, 3, crather than the power-of-ten labels used in the graph. Let’s now imagine that some quantity Y (the BMR in this example) depends on another quantity X (the mass) via the proportionality Y = CXr (1.10) where C is a constant. We say that Y scales as the rth power of X. We take the logarithm of both sides of Equation 1.10 and use the properties of logarithms: logY = log(CXr ) = logC + log(Xr ) = rlogX + logC (1.11) To interpret this equation, recall that a linear equation of the form y = mx + b graphs as a straight line with slope m and y-intercept b. By defining y = logY and x = logX, we see that Equation 1.11 is actually a linear equation. That is, a graph of logY versus logX is a straight line with slope r. Conversely, and importantly, if a graph of logY versus logX is a straight line, then Y and X obey a scaling law like Equation 1.10 and the value of the exponent r is given by the slope of the line. NOTE ▶ Spreadsheets and other graphing software that you might use to ana- lyze data usually have a “logarithmic axis” option. If you choose that option, the computer calculates and plots the logarithms of the data for you (taking logarithms is not something you have to do yourself) and then labels the axes using the power-of-ten notation shown above the axis in Figure 1.10. Using the logarithmic axis option allows you to look for functional relationships that are scaling laws. ◀ Figure 1.9 graphed the logarithm of the basal metabolic rate against the logarithm of the mass for a large number of mammals. We had no reason to suspect that these two quantities are related in any particular way, but the graph turned out to be a straight line with a slope of almost exactly 3 4. Consequently, we’ve discovered a scal- ing law telling us that a mammal’s BMR scales as the 3 4th power of its mass m; that is, BMR = Cm3/4 or, as we would usually write, BMR ∝ m3/4 . We could use the graph’s y-intercept to determine the value of the constant C, but the constant is usually less interesting than the exponent. This result has broad generality across all mammals. It is interesting that birds and insects also have metabolic rates that scale as the 3 4th power of mass. This scal- ing law really seems to be a law of biology. Why? Scaling laws, especially ones that span many orders of magnitude, tell us that there’s some underlying regularity in the physics of the organism. A simple model relating an organism’s metabolic rate to its size is based on the fact that metabolism generates heat and that heat has to be dissipated. Heat is dissipated through an organism’s surface via heat convection and radiation, so this model predicts that BMR should be proportional to the organism’s surface area. We’ve seen that surface area scales as A ∝ l2 , where l is the organism’s linear size, and thus BMR should scale with the size of an organism as BMR ∝ l2 . The organism’s mass is proportional to its volume, so mass scales as m ∝ l3 . Combining these, by eliminating l, we see that this model predicts that BMR should scale with the organism’s mass m as BMR ∝ m2/3 . That is, a geometric model in which the organism’s BMR is determined by how quickly it can dissipate heat through its surface predicts a scaling law, but one in which BMR should scale as the 2 3rd power of mass.