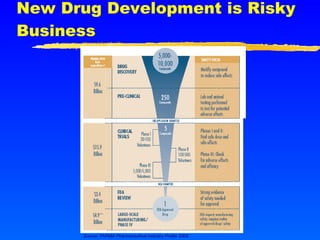
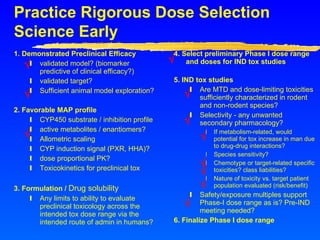
This document provides advice for small biotech companies on successful new drug development. It emphasizes that new drug development is a risky process, with many potential pitfalls that can cause drugs to fail. Some key recommendations include understanding the strengths and weaknesses of the drug, learning from previous similar drugs, rigorously selecting preclinical and clinical doses, focusing on efficacy biomarkers with potential, and considering partnerships if drug delivery technology could help. The document stresses that drug development is a marathon, not a sprint, and one should not oversell the science until it is proven.