2015-CLSA-Report-Recent-Trends-in-FDA-Med-Device-Regulation-Final
•
0 likes•122 views
Report
Share
Report
Share
Download to read offline
Recommended
Recommended
More Related Content
What's hot
What's hot (20)
Top Regulatory Trends in the Life Sciences Industry in 2017

Top Regulatory Trends in the Life Sciences Industry in 2017
Routine Diagnostics | Advance Diagnostics | Pathology Lab | Blood Test | Molq

Routine Diagnostics | Advance Diagnostics | Pathology Lab | Blood Test | Molq
What Happens After Your Device is Approved? Collecting Data in the Real World

What Happens After Your Device is Approved? Collecting Data in the Real World
Regulatory Concerns When Running Virtual/Paperless Clinical Trials

Regulatory Concerns When Running Virtual/Paperless Clinical Trials
A Pharma/CRO Partnership in the Design and Execution of Paperless Clinical Tr...

A Pharma/CRO Partnership in the Design and Execution of Paperless Clinical Tr...
Data Integrity in a GxP-regulated Environment - Pauwels Consulting Academy

Data Integrity in a GxP-regulated Environment - Pauwels Consulting Academy
2019-07 James Sullivan - Case Study - Endomechanicals

2019-07 James Sullivan - Case Study - Endomechanicals
Data integrity - Regulatory Perspective and Challenges: 

Data integrity - Regulatory Perspective and Challenges:
How eSource Solutions are Impacting Clinical Research Sites, Patients, Regula...

How eSource Solutions are Impacting Clinical Research Sites, Patients, Regula...
FDA Electronic Health Record Data in Clinical Research

FDA Electronic Health Record Data in Clinical Research
Viewers also liked
Viewers also liked (20)
Life Science Fast Track Faculty and Mentor Directory

Life Science Fast Track Faculty and Mentor Directory
Modern SEO Techniques Part 1:New SEO Methods for Medical Device Marketers

Modern SEO Techniques Part 1:New SEO Methods for Medical Device Marketers
How to Start a Med Device Startup From Your Garage - Vancouver Edition

How to Start a Med Device Startup From Your Garage - Vancouver Edition
Med Device Vendors Have Big Opportunities in Health IT Software, Services, an...

Med Device Vendors Have Big Opportunities in Health IT Software, Services, an...
Healthcare & Med Device - LiveWorx Recommended Agenda

Healthcare & Med Device - LiveWorx Recommended Agenda
Swipes pitch deck for Beta Pitch 2013 Finals in Berlin

Swipes pitch deck for Beta Pitch 2013 Finals in Berlin
The deck we used to raise $270k for our startup Castle

The deck we used to raise $270k for our startup Castle
Similar to 2015-CLSA-Report-Recent-Trends-in-FDA-Med-Device-Regulation-Final
Thomas Novelli, Medical Device Manufacturers Association - Speaker at the marcus evans Medical Device Manufacturing Summit June 2012, held in Las Vegas, NV delivered his presentation entitled Washington Update: Changes at the FDA and Health Reform ImplementationWashington Update: Changes at the FDA and Health Reform Implementation - Thom...

Washington Update: Changes at the FDA and Health Reform Implementation - Thom...marcus evans Network
Similar to 2015-CLSA-Report-Recent-Trends-in-FDA-Med-Device-Regulation-Final (20)
CHI-Report-Taking-the-Pulse-of-Medical-Device-Regulation-Innovation_Oct-2014

CHI-Report-Taking-the-Pulse-of-Medical-Device-Regulation-Innovation_Oct-2014
CLSA-BCG-Report-Tracking-FDA-Drug-Review-Performance-March-2016-Final2

CLSA-BCG-Report-Tracking-FDA-Drug-Review-Performance-March-2016-Final2
Washington Update: Changes at the FDA and Health Reform Implementation - Thom...

Washington Update: Changes at the FDA and Health Reform Implementation - Thom...
Future Challenges of Clinical Development; a View from the CRO - Hani Zaki

Future Challenges of Clinical Development; a View from the CRO - Hani Zaki
Future Challenges of Clinical Development; a View from the CRO - Hani Zaki

Future Challenges of Clinical Development; a View from the CRO - Hani Zaki
Mercer Capital's Value Focus: Laboratory Services | Mid-Year 2015

Mercer Capital's Value Focus: Laboratory Services | Mid-Year 2015
Shedding Some Light on the Insights Lurking in the PMA Database

Shedding Some Light on the Insights Lurking in the PMA Database
Influencing Payer Coverage for Advanced Genomic Testing

Influencing Payer Coverage for Advanced Genomic Testing
The value of early asset development and commercialization

The value of early asset development and commercialization
Pharma Uptoday Monthly Magazine - Volume 18; Issue: Sep 2015

Pharma Uptoday Monthly Magazine - Volume 18; Issue: Sep 2015
Regulatory Compliance in Pharmaceutical DevelopmentGL.docx

Regulatory Compliance in Pharmaceutical DevelopmentGL.docx
The 21st Century Cures Act a focus on Title III Subtitle F – Medical Device I...

The 21st Century Cures Act a focus on Title III Subtitle F – Medical Device I...
Unique Device Identification and GS1: Defining Elements in the Future of Glob...

Unique Device Identification and GS1: Defining Elements in the Future of Glob...
More from Will Zasadny
More from Will Zasadny (8)
CLSA & PwC 2017 CA Life Sciences Industry Report Final

CLSA & PwC 2017 CA Life Sciences Industry Report Final
FINAL-CHI-Report-Innovation-in-Hepatitis-C-Treatment-July-2014

FINAL-CHI-Report-Innovation-in-Hepatitis-C-Treatment-July-2014
2015-CHI-PwC-California-Biomedical-Industry-Report_Final

2015-CHI-PwC-California-Biomedical-Industry-Report_Final
2015-CLSA-Report-Recent-Trends-in-FDA-Med-Device-Regulation-Final
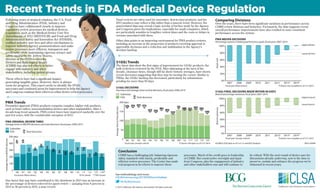
- 1. PAUL HORNSOURCE:FDA data as of 5.31.15 and BCG Analysis Recent Trends in FDA Medical Device Regulation 510(k) Trends The latest data show the first signs of improvement for 510(k) products, the bulk of devices reviewed by the FDA. After plateauing at the turn of the decade, clearance times, though still far above historic averages, have shown recent decreases suggesting that they may be turning the corner. Similar to PMAs, the 510(k) backlog has decreased, particularly for submissions pending for more than 90 days. Other data points to an improving environment for PMA product reviews, including an increase in the proportion of products receiving approval or approvable decisions and a reduction and stabilization in the Agency’s decision backlog. Submitter FDA Total decisionsXX ’13*’12*’11*’10’09*’08’07’06’05’04’03’02’01’00 ’14* *Cohorts still open as of 3.31.2014 510(k) DECISIONS Decisions and average times to final decisions,fiscal years 2000-2014 Averagetimetofinaldecision 200 150 100 50 0 days 4204 4254 3394 4322 4225 3632 3550 3853 3656 3348 4101 3880 3833 3992 3865 Panel reviews are often used for innovative, first-in-class products, and the 2013 numbers may reflect a blip rather than a nascent trend. However, the panel-related data may reveal a topic worthy of further study by the Agency and Congress given the implications, especially for small companies, which are particularly sensitive to lengthier review times and the costs or delays in revenue associated with them. Following years of strained relations, the U.S. Food and Drug Administration (FDA), industry and Congress have collaborated closely to improve regulatory review processes for medical devices. Legislation, such as the Medical Device User Fee Amendments of 2012 (MDUFA III) and Food and Drug Administration Safety and Innovation Act (FDASIA), codified industry user fees and other mechanisms to improve industry-Agency communications and make review processes more efficient, transparent and predictable while maintaining rigorous science and safety standards. Dr. Jeffrey Shuren, director of the FDA’s Center for Devices and Radiological Health (CDRH) has also led efforts to better engage with industry and other stakeholders, including patient groups. These efforts have had a significant impact, generating tangible gains. However, there is always room for progress. This report seeks to identify the FDA’s successes and continued areas for improvement to help the Agency and Congress continue their efforts to refine device review processes. One factor that may have contributed to the slowdown in 2013 was an increase in the percentage of devices referred for panel review — jumping from 8 percent in 2012 to 36 percent in 2013, a near record. 500 400 300 200 100 0 ’13*’12’11’10’09’08’07’06’05’04’03’02’01’00 ’14** AveragetimetoMDUFAdecision days Submitter FDA Total decisionsXX PMA ORIGINAL REVIEW TIMES Average times to MDUFA decisions and total decisions,fiscal years 2000-2014 *97% closed **79% closed 65 66 41 43 53 47 39 35 30 32 43 43 24 28 22 PMA Trends Premarket approval (PMA) products comprise complex, higher risk products, such as heart valves, neuromodulation devices and other implantables. After a decade-long trend upwards, PMA review times have improved markedly over the past few years, with the considerable exception of 2013. Comparing Divisions Over the years, there have been significant variations in performance across CDRH review divisions and branches. Fortunately, the data suggests recent overall performance improvements have also resulted in more consistent performance across the system. 2013*201220112010200920082007 2014* PMA MDUFA DECISIONS Percentage variance meeting performance goals,fiscal years 2007-2014 100% 80% 60% 40% 20% 0% *Cohorts not complete as of 5.31.2015 Goalsmet *Cohorts not complete as of 5.31.2015 510(k) FINAL DECISIONS MADE WITHIN 90 DAYS Branch percentage variances,fiscal years 2007-2014 2013*2012*201120102009*20082007 2014* Branchvariances 100% 80% 60% 40% 20% 0% Highest branch Lowest branch Highestdivision Lowest division See methodology and more: CALifeSciences.org/2015FDADeviceUpdate @CALifeSciences © 2015 California Life Sciences Association.All rights reserved. Conclusion CDRH has a challenging job: balancing rigorous safety standards with timely, predictable and efficient review processes. The Center has made real progress in its efforts to improve these processes. Much of the credit goes to leadership of CDRH. But constructive oversight and input from Congress, plus the engagement of industry and other stakeholders was and will continue to be critical. With the next round of device user fee discussions already underway, now is the time to preserve, sustain and enhance the progress we’ve witnessed in recent years. Fiscal year (filed cohort) Fiscal year (receipt cohort) Fiscal year (filed cohort) Fiscal year (receipt cohort)