Chemical reactions-poster-converted
•
0 likes•46 views
This document defines and describes different types of chemical reactions. It states that a chemical reaction involves the transformation of one or more substances into products with different properties. Chemical reactions are represented by equations showing reactants and products. The main types of reactions discussed are combination, decomposition, displacement, and double displacement reactions. The document also discusses endothermic and exothermic reactions, neutralization reactions between acids and bases, and the classification of oxides as basic, acidic, amphoteric, or neutral based on how they react.
Report
Share
Report
Share
Download to read offline
Recommended
Recommended
More Related Content
What's hot
What's hot (20)
Ncert class 10 - science - chapter 1 - chemical reactions and equations

Ncert class 10 - science - chapter 1 - chemical reactions and equations
CBSE CLASS 10 CHEMISTRY CHAPTER 1 CHEMICAL REACTIONS AND EQUATIONS PART 2 TYP...

CBSE CLASS 10 CHEMISTRY CHAPTER 1 CHEMICAL REACTIONS AND EQUATIONS PART 2 TYP...
CBSE CLASS 10 CHEMISTRY CHAPTER 1 CHEMICAL REACTIONS AND EQUATIONS PART 3 IN...

CBSE CLASS 10 CHEMISTRY CHAPTER 1 CHEMICAL REACTIONS AND EQUATIONS PART 3 IN...
Similar to Chemical reactions-poster-converted
Similar to Chemical reactions-poster-converted (20)
PPT-10 science.pdf,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,

PPT-10 science.pdf,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,
ncert-class10-science-chapter1-chemicalreactionsandequations-200522140131.pptx

ncert-class10-science-chapter1-chemicalreactionsandequations-200522140131.pptx
Class 10 l Science l Chemistry l Lesson 1: Chemical equations and reactions

Class 10 l Science l Chemistry l Lesson 1: Chemical equations and reactions
Recently uploaded
Mehran University Newsletter is a Quarterly Publication from Public Relations OfficeMehran University Newsletter Vol-X, Issue-I, 2024

Mehran University Newsletter Vol-X, Issue-I, 2024Mehran University of Engineering & Technology, Jamshoro
Making communications land - Are they received and understood as intended? webinar
Thursday 2 May 2024
A joint webinar created by the APM Enabling Change and APM People Interest Networks, this is the third of our three part series on Making Communications Land.
presented by
Ian Cribbes, Director, IMC&T Ltd
@cribbesheet
The link to the write up page and resources of this webinar:
https://www.apm.org.uk/news/making-communications-land-are-they-received-and-understood-as-intended-webinar/
Content description:
How do we ensure that what we have communicated was received and understood as we intended and how do we course correct if it has not.Making communications land - Are they received and understood as intended? we...

Making communications land - Are they received and understood as intended? we...Association for Project Management
Recently uploaded (20)
This PowerPoint helps students to consider the concept of infinity.

This PowerPoint helps students to consider the concept of infinity.
ICT role in 21st century education and it's challenges.

ICT role in 21st century education and it's challenges.
On National Teacher Day, meet the 2024-25 Kenan Fellows

On National Teacher Day, meet the 2024-25 Kenan Fellows
General Principles of Intellectual Property: Concepts of Intellectual Proper...

General Principles of Intellectual Property: Concepts of Intellectual Proper...
ICT Role in 21st Century Education & its Challenges.pptx

ICT Role in 21st Century Education & its Challenges.pptx
Making communications land - Are they received and understood as intended? we...

Making communications land - Are they received and understood as intended? we...
Food safety_Challenges food safety laboratories_.pdf

Food safety_Challenges food safety laboratories_.pdf
UGC NET Paper 1 Mathematical Reasoning & Aptitude.pdf

UGC NET Paper 1 Mathematical Reasoning & Aptitude.pdf
Unit-V; Pricing (Pharma Marketing Management).pptx

Unit-V; Pricing (Pharma Marketing Management).pptx
Chemical reactions-poster-converted
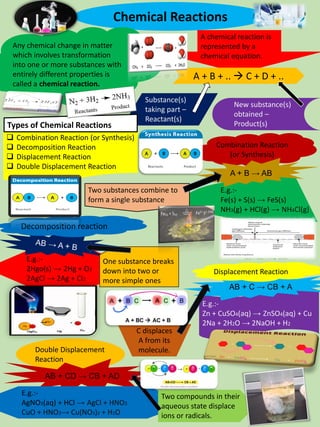
- 1. Chemical Reactions Any chemical change in matter which involves transformation into one or more substances with entirely different properties is called a chemical reaction. A chemical reaction is represented by a chemical equation. Substance(s) taking part – Reactant(s) New substance(s) obtained – Product(s) ❑ Combination Reaction (or Synthesis) ❑ Decomposition Reaction ❑ Displacement Reaction ❑ Double Displacement Reaction E.g.:- Fe(s) + S(s) → FeS(s) NH3(g) + HCl(g) → NH4Cl(g) A + B + .. → C + D + .. Types of Chemical Reactions Two substances combine to form a single substance Combination Reaction (or Synthesis) A + B → AB E.g.:- 2Hgo(s) → 2Hg + O2 2AgCl → 2Ag + Cl2 Decomposition reaction One substance breaks down into two or more simple ones E.g.:- Zn + CuSO4(aq) → ZnSO4(aq) + Cu 2Na + 2H2O → 2NaOH + H2 AB + C → CB + A C displaces A from its molecule. Displacement Reaction Double Displacement Reaction AB + CD → CB + AD E.g.:- AgNO3(aq) + HCl → AgCl + HNO3 CuO + HNO3→ Cu(NO3)2 + H2O Two compounds in their aqueous state displace ions or radicals.
- 2. A chemical reaction in which a base or alkali reacts with an acid to produce salt and water. ▪ Exothermic Reaction ▪ Endothermic Reaction A Chemical reaction in which heat is released. A Chemical reaction in which heat is absorbed. Oxides Metallic Oxides Non- Metallic Oxides Amphoteric Oxides: Oxides which are of dual nature and react with both bases and acids to produce salt and water. Eg. Zinc, Lead, Aluminium Oxide. Non-Metallic Oxides Neutralization Reaction Acid + Base/Alkali → Salt + Water Metal Reactivity Series Types of reactions based on energy exchange Basic Oxides : Most of the metallic-oxides are basic in nature and react with acids to produce salt and water. Eg. Sodium, Calcium, Magnesium Oxide. ➢ Formed on heating metals ➢ Some are formed on heating metallic, cabonates, nitrates, etc. Metallic Oxides Acidic Oxides : Most of the non-metallic oxides are acidic in nature and react with bases to produce salt and water. Eg. Carbon, Sulphur dioxide, Phosphorus Pentoxide. ➢ Formed on heating non-metals Neutral Oxides : Oxides which are neutral in nature and do not change the colour of indicators. Eg. Water, nitric, nitrous oxide.