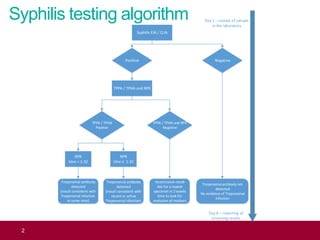
This document outlines the syphilis testing algorithm and reporting procedures used in antenatal screening. It describes a multi-step process where an initial EIA/CLIA screening test is followed by confirmatory TPPA/TPHA and RPR tests to determine if a sample is positive, negative, or inconclusive. Inconclusive results require a repeat sample in 2 weeks and may be sent to a reference laboratory. All results must be reported within 8 days to allow timely clinical evaluation and treatment if needed. The goal is to accurately detect syphilis infection status while avoiding false results and ensuring pregnant women receive appropriate care.