Recommended
More Related Content
Similar to report 2014
Similar to report 2014 (20)
GMP and cGMP Considerations Training by KLE University

GMP and cGMP Considerations Training by KLE University
GOOD LABORATORY PRACTICE - GLPMA EXPECTATIOS FOR AUDIT OF THE QUALITY ASSURAN...

GOOD LABORATORY PRACTICE - GLPMA EXPECTATIOS FOR AUDIT OF THE QUALITY ASSURAN...
New EU Requirements for Qualification & Validation

New EU Requirements for Qualification & Validation
Syllabus_B_Pharm for b pharm student studing under Rajiv Gandhi University o...

Syllabus_B_Pharm for b pharm student studing under Rajiv Gandhi University o...
More from Leandri du Plessis
More from Leandri du Plessis (7)
report 2014
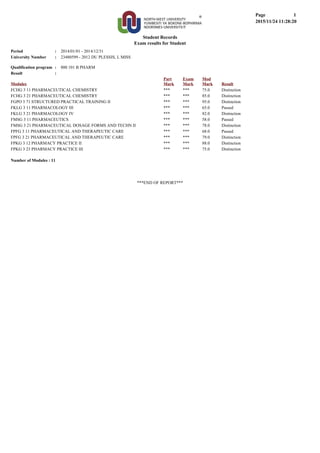
- 1. Exam results for Student Student Records 1Page 2015/11/24 11:28:20 : 2014/01/01 - 2014/12/31: 23480599 - 2012 DU PLESSIS, L MISSUniversity Number Period Qualification program : 800 101 B PHARM Result : Modules Part Mark Exam Mark Mod Mark Result FCHG 3 11 PHARMACEUTICAL CHEMISTRY *** *** 75.0 Distinction FCHG 3 21 PHARMACEUTICAL CHEMISTRY *** *** 85.0 Distinction FGPO 3 71 STRUCTURED PRACTICAL TRAINING II *** *** 95.0 Distinction FKLG 3 11 PHARMACOLOGY III *** *** 65.0 Passed FKLG 3 21 PHARMACOLOGY IV *** *** 82.0 Distinction FMSG 3 11 PHARMACEUTICS *** *** 58.0 Passed FMSG 3 21 PHARMACEUTICAL DOSAGE FORMS AND TECHN II *** *** 78.0 Distinction FPFG 3 11 PHARMACEUTICAL AND THERAPEUTIC CARE *** *** 68.0 Passed FPFG 3 21 PHARMACEUTICAL AND THERAPEUTIC CARE *** *** 79.0 Distinction FPKG 3 12 PHARMACY PRACTICE II *** *** 88.0 Distinction FPKG 3 23 PHARMACY PRACTICE III *** *** 75.0 Distinction Number of Modules : 11 ***END OF REPORT***