This laboratory manual provides instructions and procedures for experiments in a Physical Pharmacy-II Lab course. The manual was prepared by Md. Imran Nur Manik and acknowledges Sushanta Halder. The manual includes 4 experiments focused on topics like the variation of viscosity with temperature, determination of adsorption isotherms, determination of reaction velocity constants, and equilibrium constants. Precise procedures, observations, calculations and results are provided for each experiment.



![Manual: Physical Pharmacy-II Lab
Prepared By: Md. Imran Nur Manik Page 3
Lecturer; Department of Pharmacy; Northern University Bangladesh.
TOP
Part-B
1. Fix the viscometer with the burette stand vertically.
2. Pour 25 mL DW into the bulb C with a pipette.
3. Suck the liquid up near to the top of the left-limb with the help of a rubber tubing attached to it.
4. Now released the liquid to flow back into the bulb C.
5. Note the time (say t1) taken to flow the DW from A to B with a stopwatch.
6. Repeat the step 3–6 for at least 3 times and record all the efflux times.
7. Calculate the average flow time.
Part-C
1. Remove water from the viscometer and properly rinse it with the supplied sample (20% ethanol).
2. Pour supplied sample (20% ethanol) into the bulb C of the viscometer with a pipette.
3. Suck the liquid up near to the top of the left-limb with the help of a rubber tubing attached to it.
4. Now released the liquid to flow back into the bulb C.
5. Note the time (say t2) taken to flow the supplied sample (20% ethanol) from A to B with a
stopwatch.
6. Repeat the step 3-6 for at least 3 times and record all the efflux times.
7. Calculate the average flow time.
Part-D
1. Perform the processes of (part B an part C) at 25°C, 40°C and 50°C temperature.
2. Finally, calculate the relative viscosity of the supplied sample at the above mentioned
temperature.
Observation and Calculation:
Table-01: Measurement of density at different temperature
Weight of empty pycknometer, m1 (gm) = Volume of DW, Vw (mL) = Volume of Sample, V (mL) =
Temperature
Distilled Water Supplied Sample
Weight of
distilled
water and
pycnometer
m2 gm
Weight of
distilled water
mw = (m2–m1)
gm
Average
weight
of DW
Density
Vw
m
ρ
w
w
g/mL
Weight of
Sample and
pycnometer
m3 gm
Weight of
Sample
m = (m3–m1)
gm
Average
weight of
Sample
Density
Vw
m
ρ1
g/mL
25°C
40°C
50°C
Table-02: Viscosity of distilled water at the experimental temperatures.
Temperature
[°C]
Dynamic Viscosity
[mPa.s]
Kinetic Viscosity
[mm²/s]
25 0.89 0.8926
40 0.6527 0.6579
50 0.5465 0.5531](https://image.slidesharecdn.com/physicalpharmacy-iilab-180420101540/85/Physical-pharmacy-II-Lab-MANIK-5-320.jpg)



![Manual: Physical Pharmacy-II Lab
Prepared By: Md. Imran Nur Manik Page 7
Lecturer; Department of Pharmacy; Northern University Bangladesh.
TOP
Preparation of Reagents
( Full calculations required to make the reagents, for the experiment must be written down.)
1. 0.5 N 1000 mL Oxalic acid (dihydrate) solution:
Calculation: We know that W= SMV/1000
Gram equivalent weight of C2H2O4.2H2O = (126.08 ÷ 2) gm = 63.040 gm
1000 mL 0.5N H2C2O4 .2H2O= {(63.040×1000×0.5)÷1000 }gm = Y gm (for 100% pure C2H2O4.2H2O)
{N.B. Changes in purity must be taken into account to get the actual concentration}
Preparation: Completely dissolve Y gm Oxalic acid in a 1000 mL volumetric flask with a small volume
of DW. Finally adjust the volume up to 1000 mL mark by adding distilled water Q.S.
2. 0.ZN 75 mL Oxalic acid solution from 0.5 N Oxalic acid solution:
Z=0.1 , 0.2, 0.3, 0.4, 0.5
Hint: M1V1 = M2V2 [Z×100=0.5×?( V2)]
Initial values Final values
3. 0.1N 500 mL KMnO4 solution from 0.5 N Oxalic acid solution: [ Eq. No. for acid=5 ;for base=3]
Calculation: We know that W= SMV/1000
Gram equivalent weight of KMnO4 = (158.034 ÷ 5) gm = 31.607 gm
500 mL 0.5N H2C2O4 .2H2O= {(31.607×500×0.1)÷ 1000 }gm = Y gm (for 100% pure KMnO4)
{N.B. Changes in purity must be taken into account to get the actual concentration}
Preparation: Completely dissolve Y gm KMnO4 in a 500 mL volumetric flask with a small volume of
DW. Finally adjust the volume up to 500 mL mark by adding distilled water Q.S.
4. 0.05 N 100 mL KMnO4solution from Standardized ( N) KMnO4solution:
Hint: M1V1 = M2V2 [0.05×100=( N)×?( V2)]
Initial values Final values
Procedures:
1. Prepare 100 mL 0.1 N Oxalic acid solutions from 0.5 N Oxalic acid solution. [See Hint-2]
A. Standardization of KMnO4 solution by 0.1 N standard oxalic acid solutions:
i. Fill the burette with the prepared KMnO4 solution (0.1 N).
ii. Take 10 mL standard 0.1N Oxalic acid (C2H2O4.2H2O) solution in a conical flask.
iii. Add 5 mL concentrated H2SO4 in the acid solution and titrate it with the KMnO4 solution, until
the colour of the acid solution changes from colourless to faint pink.
iv. Perform another two titrations and calculate the result.
Table-1: Data for the standardization of KMnO4 solution:
No. of
observations
Volume of Oxalic acid
(V1 mL)
Volume of KMnO4 solution
(mL)
Difference
(FBR-IBR)
(mL)
Mean
volume
(V2 mL)IBR FBR
1 10
2 10
3 10](https://image.slidesharecdn.com/physicalpharmacy-iilab-180420101540/85/Physical-pharmacy-II-Lab-MANIK-9-320.jpg)
![Manual: Physical Pharmacy-II Lab
Prepared By: Md. Imran Nur Manik Page 8
Lecturer; Department of Pharmacy; Northern University Bangladesh.
TOP
B. Calculation of strength of KMnO4 solution:
We know that, V1S1 = V2S2 Here, Volume of Oxalic acid , V1 = 10 mL
S2 =
2
11
V
SV Strength of Oxalic acid ,S1 = 0.1 N
Volume of KMnO4, V2 = mL (Mean)
= Strength of KMnO4, S2= ?
= N
C. Result: The strength of KMnO4= N.
2. Prepare 0.05 N KMnO4 from the standardized KMnO4 solution.
3. Take five well cleaned, dried, 250 mL reaction bottles (/Erlenmeyer flasks). Label them from 1 to 5.
4. Place approximately 2 g of charcoal (the weight need not be exactly 2 g, but it should be known to the
nearest milligram) in each bottle (flask).
5. By means of a burette add 75, 60, 45, 30 and 15 mL of 0.5N oxalic acid; followed by 0, 15, 30, 45 and
60 mL of DW to the labelled reaction bottles, so that the total volume (75 mL) remains constant in each
bottle and the final concentration becomes 0.1, 0.08, 0.06, 0.04 and 0.02 N respectively.[See table-2]
6. Shake these bottles thoroughly nearly for half an hour (30 min) by means of a mechanical shaker and
set aside for at least 20 min to reach equilibrium.
Table-2: Amount of 0.5N Oxalic acid & Distilled Water in the reagent bottle:
Bottle No. 0.5N oxalic acid (mL) Distilled Water (mL) Conc. Of Oxalic acid Amount of charcoal (g)
01. 15 60 0.10 N 2
02. 12 63 0.08 N 2
03. 9 66 0.06 N 2
04. 6 69 0.04 N 2
05. 3 72 0.02 N 2
7. After equilibrium has been reached, filter the supernatant liquid of each of the bottle through fine dry
filter paper.
8. Collect the filtrate in properly labelled (from 1 to 5) conical flasks. Discard the first 5-10 mL of the
filtrate as a precaution against adsorption of the acid by the filter paper.
9. Take 25 mL filtrate from a labelled conical flask; in a clean conical flask with a pipette. Titrate it
against standardized 0.05 N KMnO4 solutions until a pink colour appears.
10. Repeat the titration to get concordant values.
11. Complete titrations for of each of the filtrate (obtained from five bottles with five different
concentrations).
12. From the titre values, calculate the concentration of oxalic acid remaining and later the amount of
oxalic acid adsorbed.
13. Put these values in the Freundlich equation. Plot log (x/m) against log C and draw the isotherm.](https://image.slidesharecdn.com/physicalpharmacy-iilab-180420101540/85/Physical-pharmacy-II-Lab-MANIK-10-320.jpg)
![Manual: Physical Pharmacy-II Lab
Prepared By: Md. Imran Nur Manik Page 10
Lecturer; Department of Pharmacy; Northern University Bangladesh.
TOP
Experiment No. 03 Date:
Name of the experiment: Determination of velocity constants of acid catalysed hydrolysis of
Ethyl acetate.
AIM: To determine the rate constant of the hydrolysis of Ethyl acetate using an acid as a catalyst.
Introduction:
Rate of reaction: The rate of reaction is defined as the change in the number of molecules of reacting
species per unit volume per unit time. The rate of a reaction is directly proportional to the reactant
concentrations, each concentration being raised to a certain power called the order of reaction. It is
usually taken as the rate at which the reactant disappear or the rate at which the product is formed.
For a reaction; 2A + B → products
The reaction rate with respect to A or B is determined by varying the concentration of one reactant,
keeping that of the other constant. Thus the rate of reaction may be expressed as
rate ∞[A]m
[B]n
Or, rate = k [A]m
[B]n
The proportionality constant k is called the rate constant for the reaction. Since the order of a reaction is
the sum of the powers of concentrations in the rate law; thus the order of the above reaction is (m + n).
Principle:
Hydrolysis is a chemical decomposition involving breaking of a bond and the addition of elements of
water. The use of an acid catalyst accelerates the hydrolysis. (In this hydrolysis of ester (ethyl acetate) with an
alkali (sodium hydroxide), HCl is used as catalyst to accelerate it.) The reaction rate is expressed in terms of
chemical composition of the reacting species.
Ethyl acetate upon hydrolysis in aqueous solution using a mineral acid as catalyst forms acetic acid and
ethyl alcohol.
alcoholethylacidaceticexcessacetateethyl )(
OHHCCOOHCHOHHCOOCCH 523
H
2523
Velocity of the reaction is given by
]][[)( 23 OHCOOHCHk
dt
dx
rv {Where k′=the rate constant (or specific rate constant).}
Since water is present in large excess, its active mass (molar concentration) virtually remains constant
during the course of the reaction. Therefore, its active mass gets included in the constant, and the above
equation reduces to:
][,][ 23 OHkkwhereCOOHCHk
dt
dx
Thus, the rate of the reaction is determined by one concentration term only (i.e. by a single power of the
concentration term only). Hence, the reaction is first order. Such reactions are also referred to as pseudo first
order reactions.
Let, a = initial concentration of ester (at t = 0) of the above reaction.
(a-x) = Concentration of ester remaining at time t.
)1..(....................,
)(by,drepresenteisrateThe
kdt
xa
dx
or
xak
dt
dx
)2(..............................log
3032
n,integratioOn
x)(a
a
t
.
k
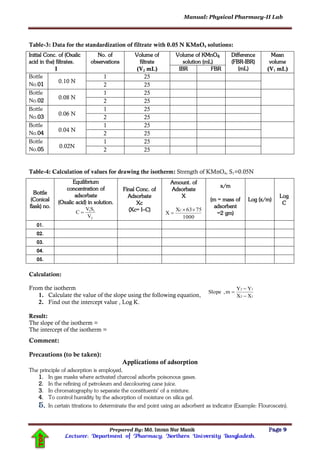
The progress of the reaction (hydrolysis of ester) is followed by removing a definite volume of the
reaction mixture, at definite intervals of time, cooling it in ice, and titrating the acetic acid formed
against alkali, which has already been standardized. The amount of alkali used is equivalent to the total
amount of hydrochloric acid present originally and the amount of acetic acid formed in the reaction.](https://image.slidesharecdn.com/physicalpharmacy-iilab-180420101540/85/Physical-pharmacy-II-Lab-MANIK-12-320.jpg)
![Manual: Physical Pharmacy-II Lab
Prepared By: Md. Imran Nur Manik Page 11
Lecturer; Department of Pharmacy; Northern University Bangladesh.
TOP
The amount of acetic acid formed (x), at definite intervals of time (t), can be obtained. The amount of
acetic acid formed, at the end of the reaction, is equivalent to the initial concentration (a) of the ester.
Suppose the volumes of the sodium hydroxide solution (titre value) required to neutralize a definite
volume of the reaction mixture are:
at the commencement of the reaction (when t=0) is Vo
after time, t is Vt
at the end of the reaction(experiment) (when t=∞) is V∞
Then:
x (amount of acetic acid formed after time t=0) is proportional to (Vt –Vo)
a (conc. of the total acetic acid formed or the conc. of ester in the beginning of the reaction or initial
concentration of ester) is proportional to (V∞ –Vo)
[a – x] (concentration of the unreacted ester at time t or concentration of ester present after time t)
is proportional to [(V∞ –Vo) – (Vt –Vo)] = (V∞–Vt)
The equation (2) expressing rate constant of the reaction becomes
)3..(....................
VV
VV
log
t
2.303
k
t
ο
The equation (3) can be rearranged as follows
)4..(....................t
2.303
k
VV
VV
log
t
ο
Equation (4) is similar to y=mx (here, c=0) and hence the velocity constant k can also be determined
from the slope (
2.303
k
m ) of the graph of
t
ο
VV
VV
log
versus t at each time.
Apparatus
1. Volumetric Flask,
2. Conical flask, Conical flask with stopper
3. Stoppered Reagent bottle
4. Beaker
5. Burette; Pipette, Thermostat
6. Graduated cylinder, funnel
7. Stirring rod, Stop watch.
Reagent
1. Ethyl acetate
2. 0.1 N Sodium Hydroxide (NaOH) Solution
3. 0.1 N KHP/ Oxalic acid Solution
4. 0.1 N HCl Solution
5. Ice/Ice cold water
6. 0.5% Phenolphthalein indicator
Preparation of Reagents
( Full calculations required to make the reagents, for the experiment must be written down.)
1. 0.1 N 250 mL NaOH solution:
Calculation: We know that W= SMV/1000
Gram equivalent weight of Na2S2O3 = (158.11 ÷ 2) gm = 79.055 gm
250 mL 0.1N Na2S2O3= {(0.1×79.055×250)÷1000 }gm = Y gm (for 100% pure Na2S2O3)
{N.B. Changes in purity must be taken into account to get the actual concentration}
Preparation: Completely dissolve Y gm Na2S2O3 in a 250 mL volumetric flask with a small volume of
DW. Finally adjust the volume up to 250 mL mark by adding distilled water Q.S.
2. 0.1N 250 mL KHP/Oxalic acid solution
Calculation: We know that W= SMV/1000
Gram equivalent weight of Na2S2O3 = (158.11 ÷ 2) gm = 79.055 gm
250 mL 0.1N Na2S2O3= {(0.1×79.055×250)÷1000 }gm = Y gm (for 100% pure Na2S2O3)
{N.B. Changes in purity must be taken into account to get the actual concentration}](https://image.slidesharecdn.com/physicalpharmacy-iilab-180420101540/85/Physical-pharmacy-II-Lab-MANIK-13-320.jpg)


![Manual: Physical Pharmacy-II Lab
Prepared By: Md. Imran Nur Manik Page 15
Lecturer; Department of Pharmacy; Northern University Bangladesh.
TOP
Experiment No. 04 Date:
Name of the experiment: Determination of the equilibrium constant (Kc) of the reaction
KI + I2 ⇌KI3.
Principle:
According to Nernst’s Distribution law, when a solute, soluble in two immiscible solvents is added to
them, gets distributed or partitioned between the separate layers of the solvents. Molecules of solute pass
from one solvent to another. Finally a dynamic equilibrium is set up. At constant temperature when the
solute is in the same molecular condition in both the solvents, there exists a constant distribution ratio
between the solvents.
If C1 denotes the concentration of the solute in one solvent and C2 the concentration in another solvent
then the Nernst’s Distribution law can be expressed as, D
2
1
K
C
C
Where, the constant KD = Distribution coefficient or Partition coefficient or Distribution ratio.
If iodine is added to a system of CCl4 and aqueous solution of KI of known concentration, it gets
distributed between the two phases. In aqueous phases, I2 combine with KI to give KI3 and exists largely
as the I3–
ion. The distribution law still applies to molecular iodine (uncombined) in the two phases.
The equilibrium will be as follows (KI and KI3 being electrolytes, are insoluble in CCl4):
KI + I2 ⇌KI3 (actually speaking I2 + I–
⇌ I3–
)
In a dilute solution the equilibrium constant of the reaction is :
)1(
]I[][I
]-[I
Kc,
]I[[KI]
][KI
Kc
2
-
3
2
3
Or
Thus knowledge of partition coefficient of I2 between CCl4 and pure water will enable us to determine
the equilibrium concentration of free iodine in aqueous KI solution in equilibrium with CCl4, provided
the concentration (conc.) of I2 in CCl4 is determined. If this is subtracted from the total conc. of I2
(determined by titration with Na2S2O3) in aqueous KI layers, the conc. of iodine that combines and hence the
conc. of KI3 formed will be obtained.
The difference of the initial conc. of KI and the conc. of KI3 gives the equilibrium conc. of KI. Knowing
the value of [I2], [KI] and [KI3] in aqueous KI layer, the equilibrium constant can be determined.
Here partition co-efficient, D = [(conc. of I2 in CCl4 layer) ÷ (conc. of I2 in aqueous layer)]
i.e. D = Corg ÷Caq
Conc. of free iodine in aqueous KI solution, [I2] = C2 ÷D
and [KI3] = C1– (C2 ÷ D)
Where C1 and C2 are the total conc. of I2 in aqueous KI layer and conc. of I2 in CCl4 layer (in equilibrium
with aqueous KI layer) respectively.
So the conc. of KI at equilibrium is:
[KI] = C– [C1– (C2 ÷ D)]
Where C = Initial conc. of KI.
Apparatus
1. Volumetric Flask
2. Conical flask; Stoppered 250 mL flasks
3. Stoppered glass bottle
4. Separating funnel
5. Burette; Pipette, Thermometer
6. graduated cylinder, funnel
7. Stirring rod, Stop watch.
Reagent
1. Saturated solution of iodine in CCl4
2. 0.1 N Sodium Thiosulfate Solution
3. 0.1 N K2Cr2O7 Solution
4. 0.1 N KI Solution
5. Concentrated HCl
6. Starch indicator](https://image.slidesharecdn.com/physicalpharmacy-iilab-180420101540/85/Physical-pharmacy-II-Lab-MANIK-17-320.jpg)
![Manual: Physical Pharmacy-II Lab
Prepared By: Md. Imran Nur Manik Page 16
Lecturer; Department of Pharmacy; Northern University Bangladesh.
TOP
Preparation of Reagents
( Full calculations required to make the reagents, for the experiment must be written down.)
1. Saturated solution of I2 in CCl4 solution:
Preparation: A small amount of iodine is added to about 200cc. of pure CCl4 in a small beaker and the
contents are stirred well to get nearly a saturated solution.
2. 10% KI solution:
Preparation: A small amount of iodine is added to about 200cc. of pure CCl4 in a small beaker and the
contents are stirred well to get nearly a saturated solution.
3. 0.01N 250 mL Sodium thiosulfate (Na2S2O3) solution
Calculation: We know that W= SMV/1000
Gram equivalent weight of Na2S2O3 = (158.11 ÷ 2) gm = 79.055 gm
250 mL 0.1N Na2S2O3= {(0.01×79.055×250)÷1000 }gm = Y gm (for 100% pure Na2S2O3)
{N.B. Changes in purity must be taken into account to get the actual concentration}
Preparation: Completely dissolve Y gm Na2S2O3 in a 250 mL volumetric flask with a small volume of
DW. Finally adjust the volume up to 250 mL mark by adding distilled water Q.S.
3. 0.01N 100 mL K2Cr2O7 solution: [ Eq. No. n=6 ]
Calculation: We know that W= SMV/1000
Gram equivalent weight of K2Cr2O7 = (294.185 ÷ 6) gm = 49.0308 gm
100 mL 0.1N K2Cr2O7= {(0.01×49.0308×100)÷1000 }gm = Y gm (for 100% pure K2Cr2O7)
{N.B. Changes in purity must be taken into account to get the actual concentration}
Preparation: Completely dissolve Y gm K2Cr2O7 in a 100 mL volumetric flask with a small volume of
DW. Finally adjust the volume up to 100 mL mark by adding distilled water Q.S.
4. 0.1 N 100 mL KI solution
Iodine 0.1 N: Weigh 40 g of potassium iodide (KI) in a 500 mL glass-stoppered flask and dissolve in 100 mL of purified
water. Let the solution come to room temperature, add 12.7 g of resublimed iodine (I2 ), restopper the flask, and swirl
the flask until the iodine is completely dissolved. Transfer the solution quantitatively to a 1 L volumetric flask, add 3
drops of hydrochloric acid (37% HCl; sp g 1.19) and dilute to 1 L with purified water. Mix thoroughly and transfer to a
glassstoppered alkali-resistant, amber-colored bottle. Iodine 0.01 N: Dilute 100 mL of 0.1 N iodine to 1 L in a volumetric
flask.
2. 0.ZN 100 mL Na2S2O3 solution from the standardized ( ) N/M Na2S2O3 solution:
Z=0.05
Hint: M1V1 = M2V2 [Z×100=( )×?( V2)]
Initial values Final values
Procedure:
1. Take thoroughly cleaned and dried six glass stoppered bottles (separating funnel). Label them from 1
to 6.
2. Now add about 50, 30 and 20 mL of the saturated solution of I2 and 0, 20 & 30 mL of pure CCl4 to the
bottles no. 1, 2 & 3 respectively. Then add 200 mL of distilled water to each bottle and stopper
tightly.[See table-01]](https://image.slidesharecdn.com/physicalpharmacy-iilab-180420101540/85/Physical-pharmacy-II-Lab-MANIK-18-320.jpg)
![Manual: Physical Pharmacy-II Lab
Prepared By: Md. Imran Nur Manik Page 17
Lecturer; Department of Pharmacy; Northern University Bangladesh.
TOP
3. To the bottle number 4, 5 & 6, add about 50, 30 and 20 mL of the saturated solution of I2
and 0, 20 and 30 mL of CCl4 respectively. Then to each bottle add 200 mL of 0.1 N KI solutions and
stopper tightly. [See table-01]
4. Now shake all the bottles from 1 to 6 vigorously for half an hour (30 minutes). Allow to stand at room
temperature for 15-20 min to secure the complete separation of the two liquid forms.
Table-01: Preparation of matures in the stoppered bottles (/separating funnel):
Bottle
No.
Volume of saturated I2 in CCl4
(mL)
Volume of Pure CCl4
(mL)
Volume of DW
(mL)
Volume of 0.1 N/M KI
(mL)
01. 50 0 200 –
02. 30 20 200 –
03. 20 30 200 –
04. 50 0 – 200
05. 30 20 – 200
06. 20 30 – 200
5. Standardize the Na2S2O3 solutions by Oxalic acid solution.
A. Standardization of Na2S2O3 solution by 0.1 N/M standard K2Cr2O7solutions:
a.Fill the burette with the prepared Na2S2O3 solution (0.1 N).
b.Take 10 mL standard 0.1N Potassium dichromate (K2Cr2O7) solution in a conical flask.
c.Add 1-2 drops of saturated starch solution and 5 mL conc. HCl into the K2Cr2O7 solution and
titrate it with the Na2S2O3 solution, until the colour changes from dark blue to colourless.
d.Perform another two titrations and calculate the result.
Table-02: Data for the standardization of KMnO4 solution:
No. of
observations
Volume of K2Cr2O7
(V1 mL)
Volume of Na2S2O3
solution (mL)
Difference
(FBR-IBR)
(mL)
Mean
volume
(V2 mL)IBR FBR
1 10
2 10
3 10
B. Calculation of strength of Na2S2O3 solution:
We know that, V1S1 = V2S2 Here, Volume of K2Cr2O7, V1 = 10 mL
S2 =
2
11
V
SV Strength of K2Cr2O7,S1 = 0.1 N
Volume of Na2S2O3, V2 = mL (Mean)
= Strength of Na2S2O3, S2= ?
= N
C. Result: The strength of Na2S2O3= N
6. Remove 50 mL of aqueous layer from 1st bottle is pipette and delivers it into a conical flask.
N. B. Take special care to prevent any contamination of CCl4 to get in the pipette. To this, add 5 mL of
10% KI solution and titrate with 0.01 M Na2S2O3 solution using 1-2 drops of starch solution as indicator.
N.B. Add starch solution just prior to the end point.](https://image.slidesharecdn.com/physicalpharmacy-iilab-180420101540/85/Physical-pharmacy-II-Lab-MANIK-19-320.jpg)
![Manual: Physical Pharmacy-II Lab
Prepared By: Md. Imran Nur Manik Page 18
Lecturer; Department of Pharmacy; Northern University Bangladesh.
TOP
7. Now insert the tip of the pipette in the lower CCl4 layer and withdraw gently in order to exclude any
drops of aqueous layer. Deliver 5 mL of CCl4 layer, then pipette it into a conical flask containing about
20 mL of 10% KI solution and titrate against 0.05 M Na2S2O3 solution using 1-2 drops of starch solution
as indicator.
N.B. Wipe all liquid from the outside of the pipette before transferring it in the titration vessel.
The contents should be shacked vigorously during titration.
The end point is dark blue to colourless.
8. Titrate the aqueous and CCl4 layer in 2nd and 3rd bottles in the similar manner. The values from the
1st
2nd
and 3rd
bottles give the partition co-efficient of I2 between water and CCl4. [Data input & Calculation table-03]
9. Remove 20 mL samples of aqueous layer and 5 mL of CCl4 layer separately by using a pipette from
bottle No. 4, 5 and 6 and titrate against 0.05 M Na2S2O3 solution respectively. [Data input & Calculation table-04 A+B]
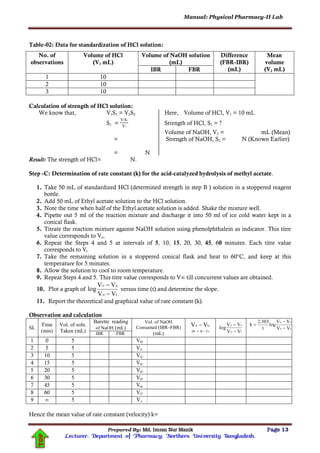
Observation and Calculation:
Temperature of the experiment:
Table-03: Determination of distribution co-efficient of I2 :
Bottle
no.
Volume of 0.01 M
Na2S2O3 solution titrate
against 50 mL aqueous
layer (V1 mL)
Volume of 0.05 M
Na2S2O3 solution titrate
against 5 mL organic (CCl4)
layer (V2 mL)
50
.010V
C
1
aq
M
5
.050V
C
2
or
M
aq
or
C
C
D
Mean
D
1
2
3
Table -04-A: Determination of concentration of I2:
Bottle
no.
Volume of 0.05 M
Na2S2O3 solution
titrated against
20 mL aqueous layer
(V3 mL)
Volume of 0.05 M
Na2S2O3 solution
titrated against
5 mL organic (CCl4)
layer (V4 mL)
Conc. of I2 and
KI3 in aq. layer
20
.050V
C
3
1
M
Conc. of free I2 in
organic layer
5
.050V
C
4
2
M
Conc. of free I2
in aq. Layer
only
D
C
][I
2
2
M
4
5
6
Table -04-B: Determination of KI3, KI and equilibrium constant K:
Bottle
no.
Conc. of KI3 in
aq. layer
D
C
C][KI
2
13
M
Conc. of KI
Equilibrium
constant
][KI][I
][KI
Kc
2
3
Mean
Kc
mol–1
dm3
250
1.0200
CKI
M
D
C
CC[KI]
2
1KI
M
4
5
6](https://image.slidesharecdn.com/physicalpharmacy-iilab-180420101540/85/Physical-pharmacy-II-Lab-MANIK-20-320.jpg)
![Manual: Physical Pharmacy-II Lab
Prepared By: Md. Imran Nur Manik Page 19
Lecturer; Department of Pharmacy; Northern University Bangladesh.
TOP
Result:
The equilibrium constant, Kc at the experimental temperature=
Comment:
Precautions (to be taken):
Titration of KMnO4 against Oxalic acid
Preparation of standard solution of Oxalic acid [250 ml M/10 (0.1 M) solution]
The molecular mass of crystalline oxalic acid is, H2C2O4.2H2O = 126
Weight of oxalic acid crystals required to prepare 1000 ml of 1 M solution = 126 g
Therefore, weight of oxalic acid required to prepare 250 ml 0.1 M solution =
Determination of strength of KMnO4 using standard solution of oxalic acid
In this titration KMnO4 is the titrant and oxalic acid is the analyte. Here, potassium permanganate is the oxidizing agent
and oxalic acid is the reducing agent. The reaction between potassium permanganate and oxalic acid is carried out in an
acidic medium because permanganate ion in the acidic medium is a very strong oxidizing agent. Acidity is introduced by
adding dil. H2SO4.
No other indicators are used to determine the endpoint, because KMnO4 acts as the indicator. Permanganate (MnO4
-
) ion
has a dark purple colour. In an acidic medium, MnO4
-
is reduced to colourless manganous (Mn2+
) ions. On reaching the
end point, the addition of the last single drop of permanganate imparts a light pink colour to the solution. The chemical
reaction that takes place during titration can be represented by the chemical equation.
Molecular equation
Ionic equation
Balanced chemical equation
From the balanced chemical equation, it is clear that 2 moles of KMnO4 reacts with 5 moles of oxalic acid.
According to the molarity equation,
n the case of KMnO4, equivalent wt is reaction specific. When KMnO4 is used in acid medium as oxidiser, 5 electrons
are gained by Mn atom. So equivalent wt of KMnO4 in acid medium = Molecular wt/no.of electrons gained in redox
reaction = 158/5 =31.6.
In alkaline or neutral medium, reaction of KMnO4 is different and Mn gains 3 electrons in redox reaction. So, for
alkaline medium redox titrations, equivalent wt of KMnO4 will be 158/3 = 52.6.](https://image.slidesharecdn.com/physicalpharmacy-iilab-180420101540/85/Physical-pharmacy-II-Lab-MANIK-21-320.jpg)